Immunohistochemistry (IHC) is a powerful and routinely used technique for the detection, localization, and scoring of cellular macromolecules in fixed tissues. Preservation and preparation of the specimen are crucial to obtain high quality results and care should be taken to select the proper fixation and embedding methods. Often, an antigen retrieval step needs also to be carried out and several protocols are available. An overview of the most common methods, their advantages and drawbacks, is presented below to help you choose the best one suited for your experiment.
Fixation
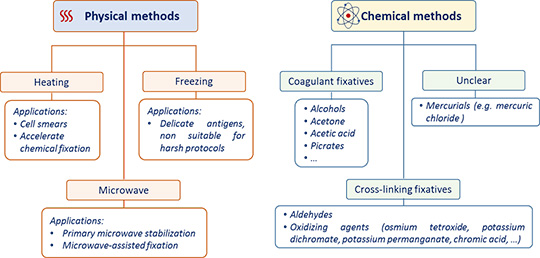
Once a tissue, organ or biopsy has been obtained, it has to be preserved as soon as possible to maintain tissue morphology, protect it from degradation, and to increase its mechanical stability for subsequent treatments. However, the different cellular and extracellular components (nucleic acids, lipids/membranes, protein, carbohydrates) will react differently to the different fixation conditions. Specific fixatives and fixation methods should, therefore, be selected based on the antigen type, its cellular localization, and of course the final aim of the experiment. A first classification of the different types of fixation can be done by distinguishing between physical or chemical methods (figure 1).
Physical methods involve the application of either heat or cold to stabilize cellular components. These include:
- Heating. Probably the simplest form of fixation. High temperature induces sample dehydration and protein precipitation; the overall morphology of the specimen is preserved but the ultrastructural organization is poorly conserved. For this reason heating is rarely used to actually fix tissues for histological studies. It is instead more commonly applied, for example, to stabilize cells smears onto a glass slide or even to accelerate other forms of fixation.
- Microwave irradiation. A specific type of fixation by heating, generated in this case by non-ionizing radiations at a frequency of 2.5 GHz. The irradiation can occur on a fresh tissue sitting in an isotonic solution (primary microwave stabilization) or, in alternative, the specimen can be irradiated while immersed in a fixing agent, such as formalin (microwave-assisted fixation). The latter method is more often used in histopathology as it can reduce fixation protocols from several hours to few minutes. The main inconvenient is the production of dangerous vapors and thus the necessity of working with appropriate instruments and protections (e.g. hoods, specifically designed microwaves, etc.).
- Cryopreservation/freeze-drying. Specimens are treated with a cryoprotective medium and immersed in liquid nitrogen, isopentane or dry ice for snap-freezing. The rapidity of the process is fundamental to avoid tissue morphological alterations caused by the formation of ice crystals.
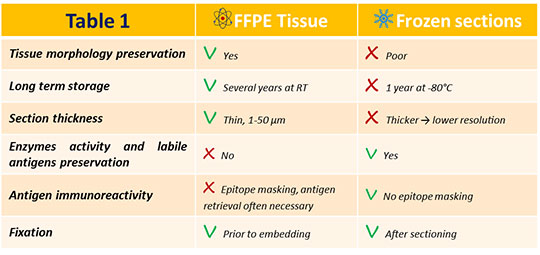
Specimens are then cut using a cryostat and finally post-fixed, usually with methanol or ethanol. Cryopreservation is particularly adapted for antigens that wouldn’t resist the chemical fixation procedure, and is therefore largely used for post-translationally modified proteins, DNA, and RNA. In addition, the whole processing is faster when compared to paraffin-embedded sections.
Table 1 displays a more detailed pros and cons list for frozen versus paraffin-embedded tissues.
The chemical fixation is obtained by using organic or inorganic solutions to preserve sample morphology for several years. Small samples can be fixed by immersion in the fixative, whereas for a rapid and homogenous fixation of an organism, or an entire organ, the perfusion of the fixing agent in the vascular system is preferred.
Chemical fixatives can be classified primarily on the basis of their mechanism of action.
- Coagulant fixatives. Also known as precipitating or denaturing fixatives for the effect they have on proteins. Typical fixing agents in these category are alcohols (e.g. methanol, ethanol) and acetone. They dehydrate the sample; thus, interfering with the stability of proteins tertiary structure and solubility, often irreversibly. These fixatives are more frequently used for frozen sections or cytologic smears. Acetone and methanol work well for example to fix large proteins, such as immunoglobulins. Acetic acid also belongs to this category but, since it coagulates nucleic acids and not proteins, it is often added to other fixative solutions to preserve nuclear content.
- Non-coagulant cross-linking fixatives. These substances create intra- and inter- molecular covalent bonds among proteins, particularly on lysine residues. Aldehydes, such as formaldehyde and glutaraldehyde, are included in this category. In its basic form formaldehyde, probably the most popular fixative in histology, is actually a gas. The liquid solution generally available in the laboratories is in fact formalin, a mixture of formaldehyde gas (37-40%) and water. To prevent its polymerization, about 10-15% of methyl alcohol is also added. Formalin penetrates slowly but deeply in tissues, and allow for the long term storage of the samples. Glutaraldehyde is less exploited for IHC staining as it causes deformation of alpha-helix structure in proteins. In addition, being larger than formaldehyde, it permeates tissue membranes more slowly. However, it fixes rapidly and gives optimal cytoplasmic and nuclear detail; it is therefore suitable for electron microscopy.
- Mercurials. They contain mercuric chloride and their fixation mechanism is unclear. Well-known fixatives such as B-5 (mercuric chloride, sodium acetate, water) and Zenker's solution (mercuric chloride, potassium dichromate, sodium sulphate, acetic acid, water) belong to this category. These fixatives penetrate relatively poorly and cause some tissue hardness, but act fast and give excellent nuclear detail. They are not used routinely anymore. Their main application is for fixation of specific samples, such as hematopoietic tissues.
- Picrates. Acting as coagulant fixatives, they react with basic proteins to form salts and are ideal to fix connective tissue. A good example of their use is the Bouin’s solution (formaldehyde, picric acid saturated aqueous solution, glacial acetic acid), specially adapted to preserve the structure of soft specimens (e.g. gastrointestinal tract).
- Oxidizing agents. They induce the formation of cross-linking, but they are essentially used as secondary fixatives because they cause extensive denaturation. Fixatives such as osmium tetroxide, potassium dichromate, potassium permanganate, and chromic acid belong to this category.
Factors influencing fixation
When establishing the protocol most adapted to your samples, different parameters need to be considered for their impact on the fixation efficiency. First, it is important to know the diffusibility of the fixing agent. That will determine its capacity to penetrate in the tissue. As previously mentioned, for example formalin and alcohols diffuse easily into the tissue, whereas glutaraldehyde is slower. To facilitate a uniform and fast
penetration, it is important to limit the thickness of the samples (≤3 mm). As with all chemical reactions, the
temperature is another critical variable factor: increasing temperature will speed up the fixation. Fixative
concentration needs also to be adjusted at the lowest possible level, as an excess might have an adverse effect and produce artefacts. For instance, the standard formalin concentration is 10%, whereas glutaraldehyde is made up at 0.25% to 4%. Finally, it is important to calculate the fixation
time. Over-fixation can produce staining artefacts or lead to epitope masking while under-fixation might cause degradation of the target as well as artefact too.
|
Figure 1: Schematic representation of the main physical and chemical fixation methods described in the article.
|
Embedding
In order to obtain thin sections for a good IHC result, the fixed sample needs to be embedded in a suitable material providing the necessary hardness to mechanically support the cutting. In most cases, this is achieved with
paraffin embedding on formalin-fixed material. Because paraffin is not soluble in water, the tissue has to undergo after fixation a progressive dehydration process by immersion in increasing alcohol concentrations. This gradual change of the environment minimizes cell damage. Finally, the sample is incubated in xylene to clear the alcohol. Paraffin is typically heated to 60°C for embedding and then allowed to harden overnight. The tissue can then be cut with a sharp blade into thin slices (1-50 µm) using a
microtome. Sections will have to be rehydrated prior to starting the immunohistochemistry staining.
In some cases, especially when working with small, soft tissues (e.g. brains dissected from little animals, such as mice or fish), it is possible to embed the specimen (fixed or unfixed) in
low melting agarose, rather than in paraffin. The protocol is faster and easier compared to paraffin embedding, as dehydration of the samples is not mandatory, no solvents are used and the agarose solidify in few minutes. Sections can be obtained using a
vibratome, an instrument similar to the microtome, characterized by a blade rapidly vibrating to cut through the tissue. Vibratome sections are on average thicker compared to microtome ones, but they often allows for sharper staining and are more adapted for the analysis of 3D detail in tissues.
|
Table 1. Paraffin-embedded versus frozen tissues pros and cons
|
Antigen or epitope retrieval
The capability of the antibody to access its epitope in the tissue can be compromised by the chemical modifications induced by the fixation (i.e. cross-linking, epitope conformation changing, etc.), especially when using formalin as fixative agent. For this reason, formalin-fixed paraffin-embedded (FFPE) samples require an
antigen retrieval step to remove the “masking” bridges and restore the immunoreactivity of the epitope. Antigen retrieval methods are divided in two categories: protease-induced epitope retrieval (PIER) and heat-induced epitope retrieval (HIER).
The
PIER approach is based on the use of
proteases, such as Proteinase K, Trypsin, and Pepsin, which are thought to cleave the peptides masking the antigen. Parameters like enzyme concentration, length of the treatment and the best temperature need to be optimized for a successful retrieval. Because the PIER can result in the alteration and the destruction of the target molecule and the morphology of the tissue, it is not used very often.
The
HIER is in fact the most common method. It consists in
heating the samples in a buffered solution, selected from several different possibilities. Even though its mechanism of action is not clear, it is known that the demasking is more efficient at higher temperatures; when the temperature is lower it is necessary to increase the length of the treatment.
Classical methods to heat the sample are the microwave oven, the pressure cooker, and the water bath. The use of microwave is today quite rare because it causes a harsh boiling, possibly leading to tissue detachment from the slide. In addition, samples are unevenly heated, resulting in a lack of staining uniformity and results reproducibility, something that is not an issue with both the pressure cooker and the water bath. When choosing one of these two methods, it is important to keep in mind that in the pressure cooker the reached temperature is higher: this certainly facilitates antigen retrieval, but can also cause alterations of tissue morphology.
Concerning the
demasking buffers available, they differ in their composition (sodium citrate, EDTA or Tris-based) and in their pH (acidic, neutral or alkaline). It seems that for most antigens, the optimal recovery is obtained with a basic buffers (pH 8-10) and that EDTA is particularly useful with over-fixed samples. However, there is not a general rule and the antigen retrieval protocol needs to be adapted and optimized case by case, taking into account appropriate controls to be able to detect false negatives/positives.
Enzo offers a complete range of products for
Immunohistochemistry detection ranging from the
antigen retrieval, to the
mounting medium to prepare your slides. Browse the complete range of Enzo’s IHC products here:
Enzo's IHC platform. Check out the amazing potential of these products by reading the related application notes:
IHC Collaborations at work. Need more advices to obtain a perfect immunostaining? Do not miss our TechNotes on the topic:
How to Select & Optimize Primary Antibodies for IHC?,
What are the different detection methods for IHC?,
What are the Common Reporter Enzymes used in IHC? Immunohistochemistry Troubleshooting Guide,
10 Tips for Successful Immunohistochemistry. For all questions and concerns, our
Technical Support Team is here to assist.











