 Courtesy of Dr Renaud Burrer (Histalim, Montpellier, France)
Courtesy of Dr Renaud Burrer (Histalim, Montpellier, France)
Immunohistochemistry (IHC) is a powerful and routinely used technique for the detection, localization, and scoring of cellular macromolecules in preserved tissues. Independent of the specific method used, the very first step of this technique is the selective binding of the primary antibody with its specific target. This interaction can then be visualized, directly or indirectly, with a chromogenic reaction catalyzed by reporter enzymes such as Horseradish Peroxidase (HRP) or Alkaline Phosphatase (AP). In the case of a
direct detection, the primary antibody (AbI) itself is labeled with HRP or AP enzymes (
Fig. 1A). However, the preferred strategy is often an
indirect detection, where the unlabeled primary antibody is recognized by a labeled secondary antibody (AbII;
Fig. 1B).
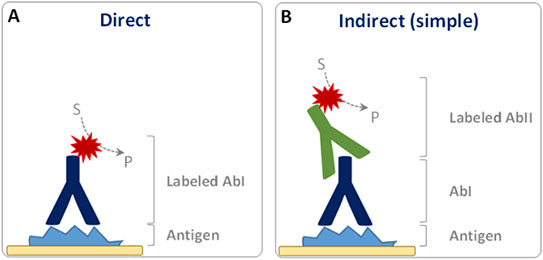
Figure 1. Schematic representation of direct (A) and indirect (B) IHC detection. S: Chromogenic substrate. P: Colored insoluble product. Reporter enzyme is indicated by the red star.
|
In both cases, a chromogenic substrate is eventually converted in a colored precipitate by the reporter enzyme, staining the tissue in correspondence to the targeted antigen. The indirect detection method is commonly used because it ensures an amplified signal and higher sensitivity, as several secondary antibodies can bind different epitopes of the primary antibody. In addition, it also allows higher flexibility as just few types of secondary antibodies can be used to detect a wide range of primary antibodies against the different antigens. A few of the most commonly used detection methods are described below.
Peroxidase/Anti-Peroxidase (PAP) Detection Method
Pioneered by Sternberger in the seventies, PAP is based on the formation of a peroxidase/anti-peroxidase complex, connected to the primary antibody via a secondary “bridge” antibody. Exploiting the bivalent properties of IgG, the PAP complex contains three peroxidase molecules and two anti-peroxidase antibodies (
Fig. 2). Compared to a simple indirect detection (reporter enzyme directly linked to the secondary antibody), this method allows for higher sensitivity because several enzyme molecules are localized per antigenic site. In addition, since the enzyme is not chemically modified, there is low risk of reducing its apparent activity.
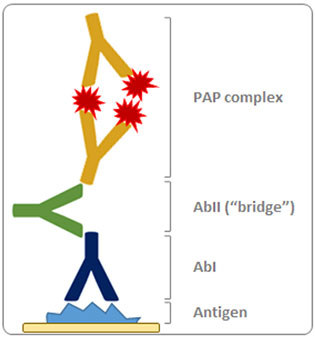
Figure 2. Schematic representation of the Peroxidase/Anti-Peroxidase (PAP) detection method. Reporter enzyme is indicated by the red star.
|
ABC (Avidin-Biotin Complex) Detection Method
This popular method is based on the almost irreversible non-covalent interaction between biotin and avidin.
Avidin is a large tetrameric glycoprotein (67-68 kDa) found in the egg-whites of amphibians, birds, and reptiles. Each subunit can bind a biotin molecule with extremely high affinity so that up to four biotins can bind to the same avidin molecule.
Biotin is a small vitamin (~244 Da, also referred to as vitamin H or B7) acting as an essential co-enzyme for mammalian carboxylases in gluconeogenesis, fatty acids synthesis, and amino acids metabolism. It is ubiquitous in mammals’ tissues in little amounts, with a slightly higher concentration in liver. Thanks to its moderate size, it can be conjugated to other macromolecules without altering its activity.
The ABC method is a three-step detection method: the primary antibody binds to the antigen in the tissue; it is in turn recognized by the biotinylated secondary antibody; complexes formed by avidin and biotinylated enzyme (ABC) can then attach to the latter. Since the avidin tetramer can bind four biotinylated biotins, large ABC lattices containing several reporter enzymes are formed, leading to a strong amplification of the signal at the antigenic site (
Fig. 3).
The elevated enzyme:antibody ratio and the consequent high sensitivity is the main advantage of this procedure, which is still one of the most widely used both in the research and in the diagnostic field. However, a few drawbacks need to be considered. For example, it can be difficult for the large ABC structures to diffuse efficiently in the tissue. Moreover, the presence of endogenous biotin is a potential source of background. With its high isoelectric point (pI=10), the avidin is positively charged and can bind negatively charged molecules (e.g. nucleic acids). In addition, its carbohydrate moiety can interact with other molecules such as lectins. These avidin-related issues can be overcome thanks to the LSAB method described below.
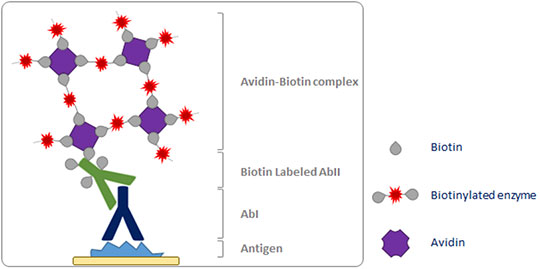
Figure 3. Schematic representation of the ABC detection method.
|
Labeled Streptavidin Biotin (LSAB) Detection Method
This technique can be considered an evolution of the previous one, as the main difference is that it uses streptavidin instead of avidin.
Streptavidin is a tetrameric protein isolated from the bacterium
Streptomyces avidinii. It has little sequence homology with avidin but their quaternary structures are very similar. Streptavidin can, in fact, bind four biotin molecules with high affinity as well, but it is not glycosylated (thereby eliminating non-specific interactions with lectin-like molecules) and it has a neutral pI (thereby limiting the risk of electrostatic binding). Thus, the LSAB method reduces background staining issues and it is ten times more sensitive than the ABC one. Similar to the ABC strategy, this is a three-step procedure: biotinylated AbII recognizes the AbI, but in this case, the streptavidin is directly conjugated to the reporter enzyme.
Figure 4 depicts IHC staining obtained with SAVIEW® PLUS detection solutions from Enzo Life Sciences. These kits are based on an improved version of the LSAB method: streptavidin molecules are labeled with a nanopolymer of the reporter enzyme thanks to a proprietary technology from Enzo’s laboratories. The SAVIEW® ready-to-use reagents enables a sensitive, enhanced streptavidin-based detection, with the highest flexibility. Choose between SAVIEW PLUS® HRP- or AP- labeled reagents, select the chromogen you prefer and get a sharp strong staining of your tissue with a manual protocol or with an automated platform.
Polymer-based Detection Method
Despite the sensitivity improvement, there is still a concern of potential false positives related to the presence of endogenous biotin with the LSAB detection method. Specific blocking procedures do exist, but this potential issue remains especially in biotin-rich tissues (e.g. liver and kidney) or in frozen tissue sections, in which biotin is better preserved compared to paraffin-embedded samples. The need for a specific, sensitive, and possibly faster method led to the development of the polymer-based detection method, which completely circumvents biotin recognition and uses polymers to increase the enzyme:AbI ratio.
One way to exploit the polymer technology is to use large dextran backbones, conjugated with up to 20 secondary antibodies and 100 enzyme molecules as represented in
Fig. 5A. This method allows for extremely sensitive detection and implies a two-step protocol, faster than the approaches described so far. One possible shortcoming is the high molecular weight of the dextran backbone, which can render the penetration of the polymer in the tissue and the interaction with some antigens (i.e. nuclear targets) difficult. The size of the polymers also reduces the enzyme density in the site of the primary antibody.
An alternative method has therefore been developed in order to get more compact antibody-enzyme complexes. This consists of polymerizing enzymes in small linear molecules and attaching these short polymers to antibodies, thus obtaining a high density of active reporters and minimizing the steric interference of the polymer (
Fig. 5B). This second generation polymer-based detection technique strongly increases the sensitivity, the specificity, and the signal intensity of the IHC, keeping a user-friendly two-step protocol.
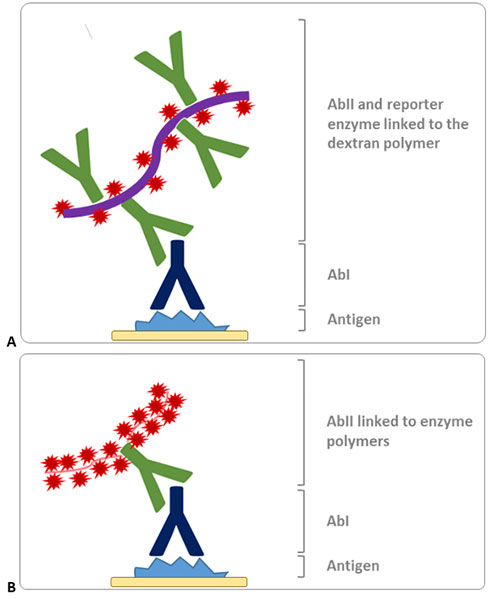
Figure 5. Schematic representation of the polymer-based detection methods. A. Dextran polymers conjugates to several reporter enzymes and secondary antibodies. B. Second generation polymer-based method, based on the generation of linear enzyme polymers, attached to the secondary antibody.
|
A similar nanopolymer-based detection technology is used for the POLYVIEW® PLUS detection solutions, developed by Enzo Life Sciences. These kits provide ready-to-use reagents, compatible with both manual and automated platforms, allowing for specific, crisp and intense staining in your IHC experiments (
Fig. 6A). If you are looking for the possibility of a superior multiplex detection, the MULTIVIEW® PLUS detection systems may be your solution (
Fig. 6B). These kits, combined with the broad palette of colors proposed by Enzo, can be used to detect up to four different targets on a single section, thus saving precious specimens and reducing reagent costs.
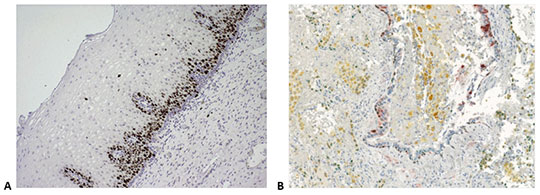
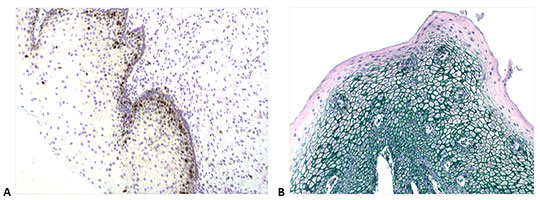
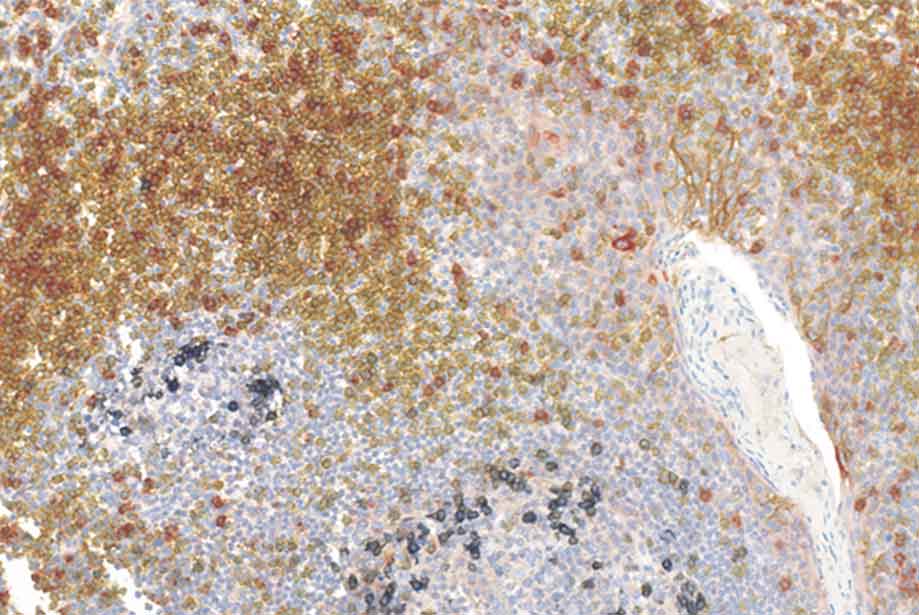
Figure 6. IHC staining obtained with Enzo’s nanopolymer-based detection solutions. A. Cervical tissue stained with anti-Ki-67 antibody (rabbit), followed by POLYVIEW® PLUS HRP (anti-rabbit) (ENZ-ACC103). B. Human lung stained with anti-CK5/6 (red, ADI-950-210), anti-Napsin A (yellow, ADI-950-170), p63 (black, ADI-950-171) and TTF-1 (green, ENZ-ACC130), using a combination of Enzo’s POLYVIEW® PLUS HRP (anti-rabbit) Reagent (ENZ-ACC103) and MULTIVIEW® PLUS (mouse-HRP/rabbit-AP) IHC Kit (ENZ-KIT181). Picture is a courtesy of Dr Renaud Burrer (Histalim, Montpellier, France).
|