The eukaryotic cell can be seen as a little factory well-organized in compartments. Each performs its task and works synchronously to ensure the variety of cellular activities necessary for life. Suppose the nucleus can be considered as the headquarters, taking the decisions and coordinating the other departments. In that case, the mitochondria represent the cell's powerhouse, providing the energy required to fuel the business in the form of ATP molecules.
According to the endosymbiont theory, it all started around two billion years ago when an anaerobic precursor of the eukaryotic cell engulfed an α-proteobacterium, capable of producing energy via the more effective aerobic respiration(1). This initiated a mutually fruitful collaboration, where the host cell could beneficiate from a more efficient ATP production while constantly providing its guest with protection, food, and oxygen. Over time, the two organisms became co-dependent and co-evolved as the eukaryotic cell began to take shape.
Mitochondria appearance has been probably essential for the evolution of multicellular organisms. These organelles are, in fact, almost ubiquitously present in eukaryotic cells, from yeast and fungi to plants and animals, with very few exceptions (e.g., red blood cells in mammals). As previously mentioned, their most popular function is in relation to the catabolism of sugars and fatty acids for ATP production. Still, they are also biosynthetic centers producing the metabolic precursors for lipids, proteins, and nucleic acids, thus contributing to cellular metabolism and bioenergetics at different levels. In addition, they exert pivotal roles in other processes central to cell function, such as calcium signaling, redox homeostasis, thermogenesis, cell proliferation, and apoptosis. Unsurprisingly, mitochondrial dysfunction is implicated in metabolic and age-related disorders and numerous pathological conditions such as neurodegenerative diseases, diabetes, ischemic injuries, cancer, etc.
Structure of mitochondria
As is often the case in biology, the mitochondria function is strictly related to their structure. Mitochondria are typically described as rod-shaped organelles, ranging in size from 0.5 to 1 µm. In reality, their shape and abundance are dynamically regulated to respond to cellular needs. Mitochondria are surrounded by two phospholipid membranes, intuitively called
outer and
inner membrane, which separate the
intermembrane space from the larger innermost matrix. The inner membrane is folded to forms several invaginations, called
cristae (Latin for crests), deeply extending into the matrix (Figure 1). Each of these defined compartments has its features and specialized functions.
- Outer mitochondrial membrane (OMM): it separates the mitochondrion from the cytoplasm. Consistent with the endosymbiont theory of mitochondria origin, its composition is similar to that of the cell membrane. It is relatively permeable as it contains several porins that allow ions and molecules smaller than ~6kDa. In a process called MOMP (Mitochondrial Outer Membrane Permeabilization), disrupting the outer membrane integrity is vital in apoptosis. It leads to the leakage of the intermembrane space content into the cytosol, including cytochrome c, a key mediator of the apoptotic caspase activation.
- Intermembrane space (IMS): despite its very limited diameter (~60 nm), it contains a large number of different proteins (~127 in humans (2)) essential for the mitochondrial function (i.e., respiratory chain, apoptosis initiation). Several complex import pathways are necessary since these proteins are mainly synthetized in the cytoplasm and not directly in the mitochondria. The IMS also acts as a buffer between the cytosol and the mitochondrial matrix by exchanging molecules and metals necessary for mitochondrial function and redox regulation (3).
- Inner membrane (IMM) and cristae delimitate the main mitochondrial sub-compartments, the intermembrane space, and the matrix. Its lipid bilayer composition is peculiar. It is rich in cardiolipin, a phospholipid with four fatty acids (rather than two), also found in bacteria membrane (once again in agreement with the endosymbiont theory). Contrarily to the OMM, the IMM represents a real barrier, regulating the transport of ions and molecules to and from the matrix through several specialized transporters. Its most prominent feature is represented by the cristae, long invaginations that significantly increase the inner membrane area. These specialized structures bear the enzymes of the oxidative phosphorylation system, the actual engine of the "powerhouse" responsible for ATP synthesis.
- Matrix: it is the innermost part of the mitochondria. It contains the mitochondrial genome (mtDNA, a peculiarity concerning the organelles of the other animal cells) and many of the metabolic enzymes required for the critical steps of cellular respiration. The mitochondrial DNA probably derives from the α-proteobacterium underlying mitochondria evolution. It is, in fact, a circular double-stranded genome, only containing 37 genes in humans, coding for two rRNAs, 22 tRNAs, and 13 proteins, all belonging to the complexes of the oxidative phosphorylation system(4). Contrary to the nuclear genome, mtDNA cannot produce all of the proteins needed for its functionality, thus relying on the material encoded in the cytoplasm and imported into the mitochondrion through its membrane system.
|
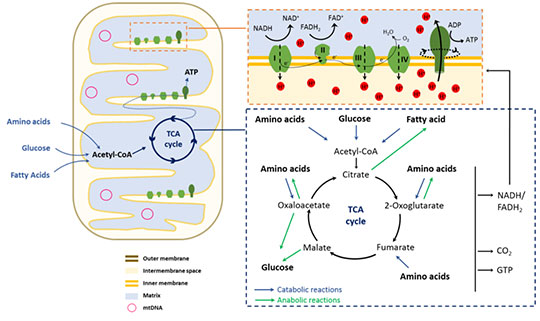
Figure 1. Schematic representation of the mitochondrial structure and principal components (on the left). A summary overview of the TCA cycle and oxidative phosphorylation is depicted on the right.
|
Cellular metabolism and mitochondria
The term metabolism indicates the sum of the chemical reactions necessary to keep an organism alive. In particular, cellular metabolism is composed of two distinct pathways, called catabolism and anabolism.
Catabolic reactions break down macromolecules (i.e., sugars, lipids, and proteins) in their building blocks (glucose, fatty acids, amino acids) to release the energy contained in their chemical bonds and store it in the form of ATP.
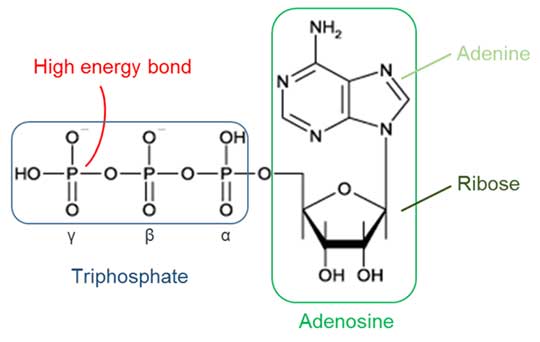
ATP is the acronym for adenosine triphosphate. It is constituted of an adenosine molecule linked to a chain of three phosphate groups (alpha, beta, or gamma, from the closest to furthest from the ribose sugar.
Figure 2). The key to the ATP energy storage potential resides in the phosphoanhydride bonds linking the phosphates β and ϒ in particular. When this is broken, the energy is again released and exploited to power the anabolic reactions.
Anabolic reactions generally correspond to the biosynthesis of macromolecules (i.e., sugars, lipids, proteins, and nucleic acids) to build or maintain cellular structures, tissues, and organs.
|
Figure 2. Representation of the ATP molecule.
|
As mentioned earlier, mitochondria are the sites of cellular respiration (aerobic catabolic reactions), and they are therefore essential in cellular bioenergetics. In fact, not only are they the major producers of ATP molecules, but they are also the site where the intermediate metabolites of the catabolic reactions can be reintegrated in anabolic pathways to synthesize new macromolecules. Cellular respiration consists of three related series of reactions.
First of all, sugars, peptides, and fatty acids are processed to generate
acetyl-CoA, a key node in metabolism, as it represents the main entry point to the subsequent mitochondrial energy conversion processes (
Figure 1).
- Glucose is the monosaccharide most typically used for energy production. It undergoes a series of ten anaerobic lytic reactions in the cytoplasm, known as glycolysis. This will generate two net ATP molecules, two molecules of NADH (an essential source of reducing equivalents for redox reactions), and two pyruvate molecules. Pyruvate is then transported into mitochondria and converted into acetyl-CoA.
- Fatty acids are transported into the mitochondria via the carnitine-based system and then catabolized by β-oxidation to generate acetyl-CoA and FADH2 and NADH cofactors eventually.
- The catabolism of some amino acids (e.g., phenylalanine, tyrosine, leucine, lysine, and tryptophan) can lead to acetyl-CoA or other intermediate molecules of the TCA cycle.
The actual "cellular respiration" starts with the
TCA cycle (tricarboxylic acid cycle), also known as the
citric acid cycle or, after his discoverer, the
Krebs cycle. As all these denominations suggest, it consists of a circular series of biochemical reactions, all taking place in the mitochondria, resulting in the generation of one molecule of GTP (equivalent to ATP in terms of energy charge) and, most importantly, three molecules of NADH, and one molecule of FADH
2, later used for the oxidative phosphorylation process. The TCA cycle initiates with the reaction of acetyl-CoA and oxaloacetate to form citrate. A succession of seven further steps eventually leads to the regeneration of oxaloacetate to restart the cycle. As anticipated, this cyclic pathway is fundamental in cellular metabolism for energy production and because intermediates can "exit" at various points and be used for biosynthetic purposes. To cite a couple of examples, the citrate is a precursor for fatty acid synthesis, and oxaloacetate is the primary precursor for gluconeogenesis in the fasting state (
Figure 1, bottom right).
Oxidative phosphorylation is the last and fundamental step in cellular respiration. It occurs through five enzymatic complexes located in the inner mitochondrial membrane, specifically at the level of the cristae (
Figure 1). The complexes I to IV constitute an
electron transport chain. The NADH and FADH
2 molecules earlier produced are the source of electrons entering the chain. A redox reaction occurring at the level of complex I (for NADH) or II (for FADH
2) allows the transfer of the electrons, releasing NAD
+ and FAD
+ and ions H
+. Electrons pass then to complexes III and IV, which eventually reduce O
2 molecules to H
2O molecules. As electrons move through the four complexes, the redox reactions release energy exploited to pump the H
+ from the matrix into the intermembrane space. This generates an
electrochemical gradient, a form of stored energy later used to drive ATP synthesis (
chemiosmosis). This energy is released with the flux of protons passing back into the matrix via the
ATP synthase, the fifth enzymatic complex of the oxidative phosphorylation chain. We can assimilate its working mechanism to a hydroelectric turbine, where the H
+ flow replaces the energy of the water, activating the
rotary motor of the enzyme: as ATP synthase rotates, it catalyzes the addition of a phosphate to ADP, capturing energy from the proton gradient as ATP (
Figure 1, top right).
It seems evident that these complex biochemical pathways are tightly regulated, often strictly interdependent, and essential for cell function. Since the mitochondria are the core of this intricate chain, mitochondrial diseases significantly impact cellular metabolism. Therefore, they are responsible for various medical conditions, ranging from less severe cases affecting a single organ to debilitating (and sometimes fatal) pathologies.
Enzo also offers a comprehensive portfolio of products to study mitochondrial dynamics in metabolic regulation. The
MITO-ID® Membrane potential detection kit is a cell-based fluorescent assay for evaluating the functional status of mitochondria, thus allowing studies on the mitochondrial activity in drug-induced toxicity, the apoptosis cascade, and other cellular and biochemical processes. For example, in a recent research article (5), this kit has been a valuable tool to characterize the molecular mechanism underlying the ZZW-115 anti-cancer effect on hepatocellular carcinoma cells. On the other hand, our MITO-ID® detection kits (
Green and
Red) allow specific mitochondrial staining, regardless of membrane potential. Therefore, this can be a tool to evaluate the mitochondrial density in the cell that could be used as an approximation to estimate cellular metabolic activity. This has been the case in an American study on obesity-induced metabolic disease, investigating the consequences of the deletion of arginase-2 in a mouse model of obesity (6). Finally, suppose you wish to identify, quantify, and characterize a specific biomarker. In that case, the
Mitochondria/Cytosol fractionation kit is an efficient and easy method to obtain enriched mitochondrial and cytosolic fractions to be used as samples for your assay. This can be useful to detect the translocation/release of factors of interest between the two fractions, as it occurs during apoptosis. With this purpose, Nemoyer
et al. (7) isolated the cytosolic fraction of lung cancer cells to observe the presence of the cytochrome c and SMAC/Diablo, indicators of apoptosis, following BMP cascade inhibition.
Do you have questions on the available tools for your research? Do you need advice to set up your experiment? Want to learn more about our portfolio? Do not hesitate to reach to our
Technical Support Team. We will be happy to assist!











