- Dual-emission dye fluoresces either green or orange depending upon mitochondrial membrane potential status
- 10X more sensitive than JC-1 with superior aqueous solubility
- True mix-and-read homogeneous assay for live cells
- Suitable for chemical/environmental toxicity screening
- Optimized for fluorescence microscopy, flow cytometry and microplate reader
- Suitable for high-throughput applications
Enzo Life Sciences MITO-ID® Membrane Potential Detection Kit includes a dual-emission cationic dye to measure the mitochondrial membrane potential (MMP) in live cells. In energized or active cells, the MITO-ID® Membrane Potential reagent rapidly accumulates as orange-fluorescent aggregates in the mitochondria due to their relative negative charge, while it exists as a green-fluorescent monomer in the cytosol. However, in cells with compromised MMP, the MITO-ID® Membrane Potential reagent exists primarily as green-fluorescent monomers throughout the cytosol and no longer exhibits orange fluorescence in the mitochondria. A widely used uncoupler of mitochondrial oxidative phosphorylation (CCCP) is provided as a positive control for monitoring loss in MMP.
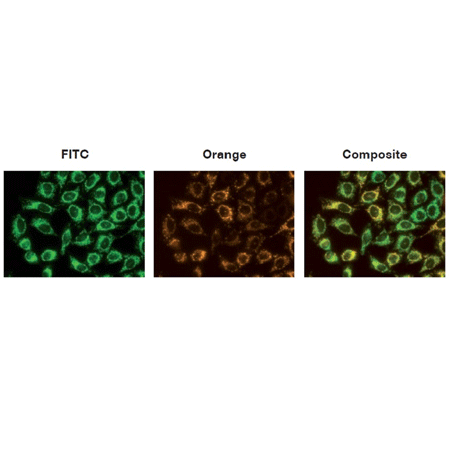
Figure 1: The mitochondria of HeLa cells were stained with MITO-ID® Membrane Potential reagent, and visualized by epifluorescence microscopy. Orange fluorescent aggregates are localized in the mitochondria (Orange channel), while green fluorescent monomers mainly stain the cytosol (FITC channel).
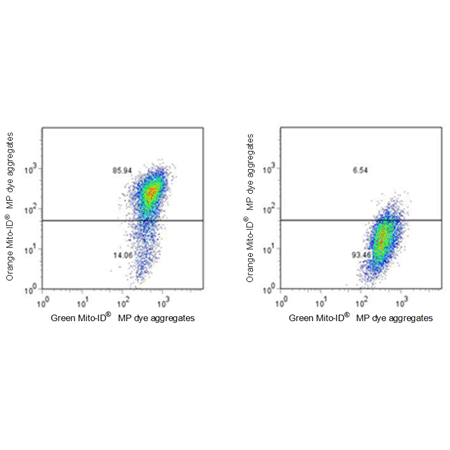
Figure 2: Flow Cytometric Analysis of Control and Treated Cells. Jurkat cells were untreated (left) and were treated with 1 μM CCCP for 15 mins (right). Cells were then stained with Enzo MITO-ID® Membrane Potential Dye, and run on a FACS Calibur instrument.
Please mouse over
Product Details
| Alternative Name: | Mitochondrial oxidative phosphorylation |
| |
| Applications: | Flow Cytometry, Fluorescence microscopy, Fluorescent detection, HTS
|
| |
| Application Notes: | The MITO-ID® Membrane Potential Detection Kit has been optimized for measurement of mitochondria membrane potential (MMP) and cell viability by conventional fluorescence microscopy and flow cytometry. |
| |
| Quality Control: | In fluorescence microscopy assay, MITO-ID® Membrane Potential dye is a dual-emission probe emitting in the green channel in the cytosol and in the orange channel within the mitochondria. When a perturbing drug such as CCCP is added, the dye exists primarily as green-fluorescent monomers in the cytosol and no longer exhibits orange fluorescence in the mitochondria. Necrosis detection reagent detects dead cells shown in the red channel. |
| |
| Handling: | Protect from light. Avoid freeze/thaw cycles. |
| |
| Shipping: | Dry Ice |
| |
| Short Term Storage: | -20°C |
| |
| Long Term Storage: | -80°C |
| |
| Contents: | MITO-ID® MP Detection Reagent
Necrosis Detection Reagent
CCCP Control
10X Assay Buffer 1
50X Assay Buffer 2 |
| |
| Scientific Background: | Cell-based assays for evaluating the functional status of mitochondria are emerging as useful tools for elucidating the role of mitochondrial activity in drug-induced toxicity, the apoptosis cascade and other cellular and biochemical processes. The loss of the mitochondrial membrane potential (MMP) is often associated with early stages of apoptosis. The collapse of MMP coincides with the opening of the mitochondrial permeability transition pores, leading to the release of cytochrome C into the cytosol, which in turn triggers other downstream events in the apoptotic cascade. |
| |
| Technical Info/Product Notes: | The MITO-ID® Membrane potential detection kit is a member of the CELLESTIAL® product line, reagents and assay kits comprising fluorescent molecular probes that have been extensively benchmarked for live cell analysis applications. CELLESTIAL® reagents and kits are optimal for use in demanding cell analysis applications involving confocal microscopy, flow cytometry, microplate readers and HCS/HTS, where consistency and reproducibility are required.
Toxicology Application Note:
Use of 3D Cultured Human iPSC-Derived Hepatocytes for Long-Term Hepatotoxicity Studies
Monitor Mitochondrial Membrane Potential in Cancer Cell Lines with a Dual-emission Fluorescent Dye |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
Discovery of the 3-Amino-1,2,4-triazine-Based Library as Selective PDK1 Inhibitors with Therapeutic Potential in Highly Aggressive Pancreatic Ductal Adenocarcinoma: D. Carbone, et al.; Int. J. Mol. Sci.
24, 3679 (2023),
Abstract;
Gene signature predicting recurrence in oral squamous cell carcinoma is characterized by increased oxidative phosphorylation: J.K. Noh, et al.; Mol. Oncol.
17, 134 (2023),
Abstract;
Cu(I) and Cu(II) Complexes Based on Lonidamine-Conjugated Ligands Designed to Promote Synergistic Antitumor Effects: F.D. Bello, et al.; Inorg. Chem.
61, 4919 (2022),
Abstract;
Mitochondrial membrane potential-enriched CHO host: a novel and powerful tool for improving biomanufacturing capability: L. Chakrabarti, et al.; MAbs
14, 2020081 (2022),
Abstract;
New glycoconjugation strategies for Ruthenium(II) arene complexes via phosphane ligands and assessment of their antiproliferative activity: D. Iacopini, et al.; Bioorg. Chem.
126, 105901 (2022),
Abstract;
New tricyclic systems as photosensitizers towards triple negative breast cancer cell: M. Barreca, et al.; Arch. Pharm. Res.
45, 806 (2022),
Abstract;
Role of caspase-8 and/or-9 as biomarkers that can distinguish the potential to cause toxic-and immune related-adverse event, for the progress of acetaminophen-induced liver injury: T. Noda, et al.; Life Sci.
294, 120351 (2022),
Application(s): Microplate reader,
Abstract;
Unveiling the Potential of Innovative Gold(I) and Silver(I) Selenourea Complexes as Anticancer Agents Targeting TrxR and Cellular Redox Homeostasis: M.D. Franco, et al.; Chemistry
28, e202201898 (2022),
Abstract;
Altered inflammatory response in FMRP-deficient microglia: J.M. Parrott, et al.; iScience
24, 103293 (2021),
Application(s): Fluorescence microscopy,
Abstract;
Full Text
ApoE4 impairs neuron-astrocyte coupling of fatty acid metabolism: G. Qi, et al.; Cell. Rep.
34, 108572 (2021),
Application(s): Microplate reader,
Abstract;
Full Text
Botrytis cinerea methyl isocitrate lyase mediates oxidative stress tolerance and programmed cell death by modulating cellular succinate levels: L. Oren-Young, et al.; Fungal Genet. Biol.
146, 103484 (2021),
Application(s): Conidia (fungus spore); microscopy,
Abstract;
Copper (II) complexes containing natural flavonoid pomiferin show considerable in vitro cytotoxicity and anti-inflammatory effects: J. Vanco, et al.; Int. J. Mol. Sci.
22, 7626 (2021),
Application(s): Flow cytometry,
Abstract;
Full Text
Depletion of mitochondrial components from extracellular vesicles secreted from astrocytes in a mouse model of fragile X syndrome: B.G. Ha, et al.; Int. J. Mol. Sci.
22, 410 (2021),
Application(s): Fluorescence microscopy,
Abstract;
Full Text
Inhibiting autophagy targets human leukemic stem cells and hypoxic AML blasts by disrupting mitochondrial homeostasis: K.M. Dykstra, et al.; Blood Adv.
5, 2087 (2021),
Abstract;
Selective striatal cell loss is ameliorated by regulated autophagy of the cortex: K. Cho & G.W. Kim; Life Sci.
282, 119822 (2021),
Application(s): Flow cytometry,
Abstract;
Adverse effects of Δ9-tetrahydrocannabinol on neuronal bioenergetics during postnatal development: J. Beiersdorf, et al.; JCI Insight
5, e135418 (2020),
Application(s): Cortical neurons; microscopy,
Abstract;
Full Text
Cortical cells are altered by factors including bone morphogenetic protein released from a placental barrier model under altered oxygenation: V.H.L. Leinster, et al.; Neuronal Signal.
4, NS20190148 (2020),
Application(s): Flow cytometry,
Abstract;
Full Text
Novel Pt(IV) Prodrugs Displaying Antimitochondrial Effects: L. Tabrizi, et al.; Mol. Pharm.
17, 3009 (2020),
Application(s): MCF-7 cells; microscopy; 96-well plate,
Abstract;
Targeting Mitochondrial Complex I Overcomes Chemoresistance in High OXPHOS Pancreatic Cancer: R. Masoud, et al.; Cell Rep. Med.
1, 100143 (2020),
Application(s): Pancreatic ductal adenocarcinoma cells; microscopy,
Abstract;
Full Text
Targeting NUPR1 with the small compound ZZW-115 is an efficient strategy to treat hepatocellular carcinoma: W. Lan, et al.; Cancer Lett.
486, 8 (2020),
Application(s): Flow cytometry,
Abstract;
Dissecting the anticancer mechanism of trifluoperazine on pancreatic ductal adenocarcinoma: C. Huang, et al.; Cancers (Basel)
11, 1869 (2019),
Application(s): Flow cytometry,
Abstract;
Full Text
Elimination of protein aggregates prevents premature senescence in human trisomy 21 fibroblasts: N. Nawa, et al.; PLoS One
14, e0219592 (2019),
Abstract;
Full Text
Mitochondrial lipid droplet formation as a detoxification mechanism to sequester and degrade excessive urothelial membranes: Y. Liao, et al.; Mol. Biol. Cell
30, 2969 (2019),
Abstract;
Full Text
Mitochondrial membrane potential identifies cells with high recombinant protein productivity: L. Chakrabarti, et al.; J. Immunol. Methods
464, 31 (2019),
Application(s): Flow cytometric analysis of mitochondrial membrane potential,
Abstract;
NK-cell responses are biased towards CD16-mediated effector functions in chronic hepatitis B virus infection: A. Schuch, et al.; J. Hepatol.
70, 351 (2019),
Application(s): Flow cytometry,
Abstract;
Zn-doped CuO nanocomposites inhibit tumor growth by NF-κB pathway cross-linked autophagy and apoptosis: H. Xu, et al.; Nanomedicine (Lond.)
14, 131 (2019),
Abstract;
A fragment of adhesion molecule L1 is imported into mitochondria, and regulates mitochondrial metabolism and trafficking: K. Kraus, et al.; J. Cell. Sci.
131, jcs210500 (2018),
Abstract;
Comparative gene expression analysis in WM164 melanoma cells revealed that β-β-Dimethylacrylshikonin leads to ROS generation, loss of mitochondrial membrane, and autophagy induction: N. Kretschmer, et al.; Molecules
23, 2823 (2018),
Application(s): Microplate reader,
Abstract;
Full Text
Inactivation of NUPR1 promotes cell death by coupling ER-stress responses with necrosis: P. Santofimia-Castano, et al.; Sci. Rep.
8, 16999 (2018),
Application(s): Flow cytometry,
Abstract;
Full Text
Leptospermum flavescens Sm. protect pancreatic β cell function from streptozotocin involving apoptosis and autophagy signaling pathway in in vitro and in vivo case study: A.F.A. Hidayat, et al.; J. Ethnopharmacol.
226, 120 (2018),
Application(s): Flow cytometry,
Abstract;
Novel β-phenylacrylic acid derivatives exert anti-cancer activity by inducing Src-mediated apoptosis in wild-type KRAS colon cancer: S.J. Kim, et al.; Cell Death Dis.
9, 877 (2018),
Abstract;
Full Text
Burn serum stimulates myoblast cell death associated with IL-6-induced mitochondrial fragmentation: A. Sehat, et al.; Shock
48, 236 (2017),
Application(s): cell viability after burn,
Abstract;
Full Text
Decreased Poly(ADP-Ribose) Polymerase 1 Expression Attenuates Glucose Oxidase-Induced Damage in Rat Cochlear Marginal Strial Cells: Y. Zhang, et al.; Mol. Neurobiol.
53, 5971 (2016),
Abstract;
Effects of tributyltin chloride on cybrids with or without an ATP synthase pathologic mutation: E. Lopez-Gallardo, et al.; Environ. Health Perspect.
124, 1399 (2016),
Abstract;
Mutant p53 inhibits miRNA biogenesis by interfering with the microprocessor complex: F. Garibaldi, et al.; Oncogene
35, 3760 (2016),
Abstract;
Overexpression of HPV16 E6* alters β-integrin and mitochondrial dysfunction pathways in cervical cancer cells: W. Evans, et al.; Cancer Genomics Proteomics
13, 259 (2016),
Abstract;
Oxidative Stress Induced by Pt(IV) Pro-drugs Based on the Cisplatin Scaffold and Indole Carboxylic Acids in Axial Position: D. Tolan, et al.; Sci. Rep.
6, 29367 (2016),
Application(s): Assay of mitochondrial membrane potential (ΔΨm),
Abstract;
Full Text
Diallyl trisulfide induces apoptosis in Jurkat cells by the modification of cysteine residues in thioredoxin: K. Watanabe, et al.; Biosci. Biotechnol. Biochem.
78, 1418 (2014),
Application(s): Flow cytometry measurements on Jurkat cells,
Abstract;
Expanding the clinical phenotypes of MT-ATP6 mutations: E. Lopez-Gallardo, et al.; Hum. Mol. Genet.
23, 6191 (2014),
Abstract;
Proteome variations in pancreatic stellate cells upon stimulation with pro-inflammatory factors: A.J. Marzog, et al.; J. Biol. Chem.
288, 32517 (2013),
Application(s): Apoptotis assay in pancreatic stellate cells,
Abstract;
Full Text
Utilization of fluorescent probes for the quantification and identification of subcellular proteomes and biological processes regulated by lipid peroxidation products: T.D. Cummins, et al.; Free Radic. Biol. Med.
59, 56 (2013),
Application(s): Cell Culture,
Abstract;
Full Text
CPTH6, a thiazole derivative, induces histone hypoacetylation and apoptosis in human leukemia cells: D. Trisciuoglio, et al.; Clin. Cancer Res.
18, 475 (2012),
Application(s): Detection of membrane potential in AML and solid tumor cell lines by flow cytometry,
Abstract;
Full Text
Docosahexaenoic acid-induced unfolded protein response, cell cycle arrest, and apoptosis in vascular smooth muscle cells are triggered by Ca²⁺-dependent induction of oxidative stress: S. Crnkovic, et al.; Free Radic. Biol. Med.
52, 1786 (2012),
Application(s): Detection of membrane potential in human pulmonary artery smooth muscle cells by flow cytometry,
Abstract;
Full Text
Ectopic ATP synthase blockade suppresses lung adenocarcinoma growth by activating the unfolded protein response: H.Y. Chang, et al.; Cancer Res.
72, 4696 (2012),
Application(s): Mitochondrial membrane potential in lung carcinoma cells by fluorescence microscopy,
Abstract;
Full Text
Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells: M. Hefti, et al.; J. Neurooncol.
108, 443 (2012),
Abstract;
General Literature References
High throughput fluorescence assays for the measurement of mitochondrial activity in intact human neuroblastoma cells: A.J. Woollacott & P.B. Simpson; J. Biomol. Screen
6, 413 (2001),
Abstract;
A New Method for the Cytofluorometric Analysis of Mitochondrial Membrane Potential Using the J-Aggregate Forming Lipophilic Cation 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine Iodide (JC-1): A. Cossarizza, et al.; Biochem. Biophys. Res. Commun.
197, 40 (1993),
Abstract;
Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate forming lipophilic cation JC-1: S.T. Smiley, et al.; PNAS
88, 3671 (1991),
Full Text
Related Products