Product Details
| Host: | Rabbit |
| |
| Immunogen: | Recombinant ADRM1 (Thr2-Asp407) expressed in E.coli |
| |
| UniProt ID: | Q16186 |
| |
| Species reactivity: | Human
|
| |
| Applications: | ICC, IHC, WB
|
| |
| Recommended Dilutions/Conditions: | Immunocytochemistry (5-20 µg/mL)
Immunohistochemistry (5-20 µg/mL)
Western blot (0.5-2 µg/mL)
Optimal conditions must be determined individually for each application. |
| |
| Formulation: | Liquid. In 0.01M PBS, pH 7.4, containing 0.05% Proclin-300 and 50% glycerol. |
| |
| Shipping: | Blue Ice |
| |
| Long Term Storage: | -20°C |
| |
| Scientific Background: | Originally described as an interferon-γ-inducible, heavily glycosylated membrane protein of 110 kDa which regulates cell adhesion, recent studies have identified Adrm1 as a novel component of the regulatory ATPase complex (19S) of the 26S proteasome. Contrary to previous studies, it is now demonstrated that Adrm1 is found only as a 42 kDa protein, corresponding to the mass of the nonglycosylated peptide chain, and that it cannot not be induced in HeLa cells with interferon. Database searches suggest that the budding yeast proteasome subunit Rpn13 may be a distantly related orthologue of Adrm1, although there is only 25% identity in the stretch of aligned residues. The budding yeast orthologue is also much shorter than human Adrm1 and lacks most of the adhesion regulating molecule (ARM) domain situated in the central part of the protein with, so far, no function having been ascribed to the ARM domain. However, among mammals, Adrm1 is phylogenetically well preserved with 97% identity between the human and murine proteins. Adrm1 is present in approximately stoiciometric in HeLa cells although some cells lack Adrm1 entirely. Rpn13 deficient mutants display no obvious phenotype and, similarly, knock-down of Adrm1 in HeLa cells shows no effect on amount of proteasomes, or on degradation of bulk protein, or accumulation of polyubiquitinylated proteins. It has been demonstrated that Uch37 (one of the three principal deubiquitinylating enzymes) binds through Adrm1, which in turn is bound to the S1/Rpn2 subunit of the 19S complex. Adrm1 binds the carboxy-terminal tail of Uch37, a region that is distinct from the UCH catalytic domain, which inhibits Uch37 activity. Adrm1 appears to be a stable protein and all material in cell extracts is found in proteasomes. Consequently Adrm1 is most likely a subunit, or an associated protein, of the proteasome depending on definition. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
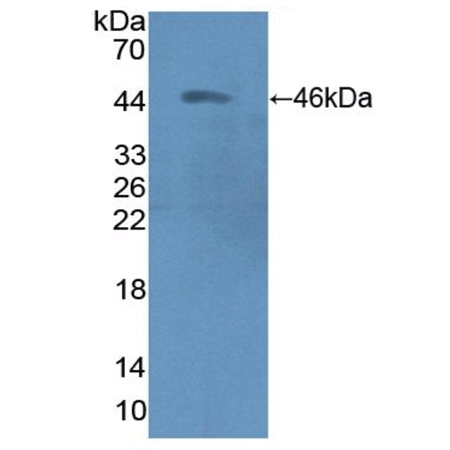
Figure 1. Western blot analysis using 19S Proteasome (ADRM1), pAb (Prod.No. ENZ-ABS296)
Please mouse over
Product Literature References
Open-gate mutants of the mammalian proteasome show enhanced ubiquitin-conjugate degradation: W.H. Choi, et al.; Nat. Commun.
7, 10963 (2016),
Application(s): Western blot,
Abstract;