| Alternative Name: | Ubiquitin C-terminal hydrolase L3 |
| |
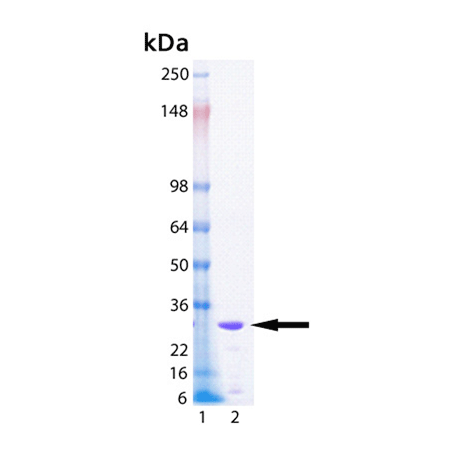
| MW: | 26.2 kDa |
| |
| Source: | Produced in E. coli. |
| |
| UniProt ID: | P15374 |
| |
| GenBank ID: | NM_006002 |
| |
| Formulation: | Liquid. In 50mM TRIS, pH 7.6, containing 1mM dithiothreitol (DTT). |
| |
| Purity: | ≥90% (SDS-PAGE) |
| |
| Specific Activity: | >1000 pmol/min/μg. Determined at 25°C with 1μM ubiquitin-AMC (Prod. No. BML-SE211) as substrate and UCH-L3 at 20pM (0.58ng/ml; see protocol). |
| |
| Application Notes: | Useful tool to study UCH-L3 kinetics and regulation, screen for potential inhibitors and activators. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Use/Stability: | As supplied, the enzyme is stable on ice for several hours. Activity is stable to at least 6 freeze/thaw cycles. (snap freezing in a dry/ice ethanol bath or liquid nitrogen). |
| |
| Scientific Background: | Ubiquitin C-terminal hydrolases (UCHs) are a family of cysteine hydrolases that catalyze the hydrolysis of amides, esters and thioesters of the C-terminus of ubiquitin. UCH-L3 is a member of the lower molecular weight group of UCHs involved in the hydrolysis of small C-terminal derivatives of ubiquitin that form non-specifically during the process of protein ubiquitinylation. |
| |
| Protocol: | Microplate Fluorimetric Assay of UCH-L3 (Cat. # BML-UW9745) with Ubiquitin-AMC (Cat. # BML-SE211)
Components of Assay:
Assay Buffer: 50 mM HEPES/NaOH, pH 7.8, 0.5 mM EDTA, 1 mM DTT, 0.1 mg/ml ovalbumin
UCH-L3 (BML-UW9745) Dilute to 20-fold the desired final concentration in cold Assay Buffer (e.g. 400 pM/11.6 ng/ml), and keep on ice until use.
Ubiquitin-AMC (BML-SE211) 0.542 mg/ml (62 μM) solution in 50 mM sodium acetate, pH 7.0. Prepare a dilution in Assay Buffer which is 20-fold the desired assay concentration (see step 3 below).
AMC Standard (BML-KI107): 30 μM solution in 50 mM HEPES/NaOH, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA, 10% glycerol. Prepare a series of dilutions in Assay Buffer (0-1.0 μM) for use in preparation of a standard curve.
½ Volume 96-well white micro-plate (BML-KI110)
Microplate-reading Fluorimeter: Reader should be capable of excitation at a wavelength in the range of 350-380 and detection of emitted light in the range of 440-460 nm.
Assay Procedure :
1) Add 90 μl of Assay Buffer to the desired number of microplate wells and warm to assay temperature (e.g. 25°C).
2) Add 5 μl of diluted UCH-L3 and incubate for 30 min. to allow time for DTT-mediated activation of the enzyme.
3) Start the reaction by adding 5 μl of the 20x ubiquitin-AMC and mixing well. The Km of UCH-L3 for ubiquitin-AMC has been determined to be 39 nM2 and assays are typically performed at final concentrations in the range of 0-1 μM.
4) Monitor the progress of the reaction by measuring the fluorescence of the AMC cleaved from the ubiquitin-AMC. For example, read fluorescence (Ex. 350-380 nm; Em. 440-460) at 1 min. intervals for 10 min.
5) Determine the fluorescence in wells filled with 100 μl of a series of dilutions of AMC in Assay Buffer (0-1 μM). Plot AMC concentration (μM, y-axis) as a function of fluorescence (arbitrary fluorescence units, AFU, x-axis).
6) Data analysis: A conversion factor relating fluorescence to AMC concentration may be determined from the slope of the line (μM/AFU) obtained in step 5). Initial rates of ubiquitin-AMC cleavage by UCH-L1 may be determined from the slope (AFU/min) of the early, linear part of the reaction progress curve. A rate in, for example, pmol/min, can then be calculated by multiplying the initial slope of the progress curve by the slope of the calibration curve and the reaction volume (100 μl):
Rate (pmol/min) = (AFU/min) x (μM/AFU) x (100 μl) |
| |
| Regulatory Status: | RUO - Research Use Only |
| |