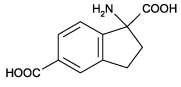
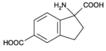
Potent and selective ligand for group I mGluRs. Shows no effect on group II or group III mGluRs. Has no effect on ionotropic glutamate receptors.
Product Details
| Alternative Name: | (±)-1-Aminoindane-1,5-dicarboxylic acid, UPF 523 |
| |
| Formula: | C11H11NO4 |
| |
| MW: | 221.2 |
| |
| CAS: | 168560-79-0 |
| |
| Purity: | ≥96% |
| |
| Solubility: | Soluble in water. |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | +4°C |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
1-Aminoindan-1,5-dicarboxylic acid and (S)-(+)-2-(3'- carboxybicyclo[1.1.1] pentyl)-glycine, two mGlu1 receptor-preferring antagonists, reduce neuronal death in in vitro and in vivo models of cerebral ischaemia: D.E. Pellegrini-Giampietro, et al.; Eur. J. Neurosci.
11, 3637 (1999),
Abstract;
Evaluation of agonists and antagonists acting at Group I metabotropic glutamate receptors in the thalamus in vivo: T.E. Salt, et al.; Neuropharmacology
38, 1505 (1999),
Abstract;
Induction of LTD by activation of group l mGluR in the denate gyrus in vitro: N. Camodeca, et al.; Neuropharmacology
38, 1597 (1999),
Abstract;
Metabotropic G-protein-coupled glutamate receptors as therapeutic targets: R. Pellicciari & G. Costantino; Curr. Opin. Chem. Biol.
3, 433 (1999), (Review),
Abstract;
Pharmacological agents acting at subtypes of metabotropic glutamate receptors: D.D. Schoepp, et al.; Neuropharmacology
38, 1431 (1999), (Review),
Abstract;
Class I mGlu receptor antagonist 1-aminoindan-1,5-dicarboxylic acid blocks contextual but not cue conditioning in rats: K.S. Nielsen, et al.; Eur. J. Pharmacol.
326, 105 (1997),
Abstract;
mGluR modulation of post-traumatic neuronal death: role of NMDA receptors: A.G. Mukhin, et al.; Neuroreport
8, 2561 (1997),
Abstract;
Pharmacological characterization of 1-aminoindan-1,5-dicarboxylic acid, a potent mGluR1 antagonist: F. Moroni, et al.; J. Pharmacol. Exp. Ther.
281, 721 (1997),
Abstract;
1-Aminoindan-1,5-dicarboxylic acid: a novel antagonist at phospholipase C-linked metabotropic glutamate receptors: R. Pellicciari, et al.; J. Med. Chem.
38, 3717 (1995),
Abstract;