| Alternative Name: | Endoplasmin, Tra1, Hsp90B1, Gp96 |
| |
| Clone: | 9G10 |
| |
| Host: | Rat |
| |
| Isotype: | IgG2a |
| |
| Immunogen: | Native chicken Grp94. |
| |
| UniProt ID: | P08110 |
| |
| GenBank ID: | M14772 |
| |
| Source: | Purified from ascites. |
| |
| Species reactivity: | Human, Mouse, Rat
Bovine, Chicken, Dog, Guinea pig, Hamster, Monkey, Porcine, Rabbit, Sheep, Xenopus
|
| |
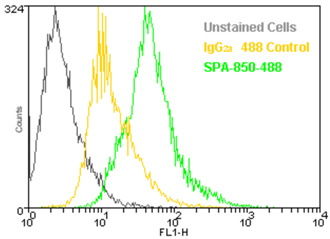
| Applications: | Flow Cytometry
|
| |
| Recommended Dilutions/Conditions: | Flow Cytometry (1:20)
Suggested dilutions/conditions may not be available for all applications.
Optimal conditions must be determined individually for each application. |
| |
| Application Notes: | Detects a band of ~94kDa by Western blot. |
| |
| Purity Detail: | Protein G affinity purified. |
| |
| Formulation: | Liquid. In PBS, pH 7.2, containing 0.09% sodium azide. |
| |
| Handling: | Avoid freeze/thaw cycles. Protect from light. |
| |
| Shipping: | Blue Ice |
| |
| Long Term Storage: | +4°C |
| |
| Scientific Background: | Glucose-regulated protein 94 (Grp94, gp96), an abundant resident endoplasmic reticulum (ER) lumenal stress protein, belongs to the Hsp90 family of molecular chaperones along with cytosolic Hsp90. Grp94 and such other resident soluble proteins of the ER as the Ca 2+ binding protein subfamily (CaBP, CaBPI, CaBP2 and calreticulin) possess the C-terminal tetrapeptide Lys-Asp-Glu-Leu (KDEL), a sorting signal considered responsible for the retention of these proteins in the pre-Golgi compartments. Stress conditions such as glucose starvation and heat shock which promote protein misfolding or unfolding increase Grp94 expression. In addition to a homeostatic role in protein folding and assembly, Grp94 can function in the intracellular trafficking of peptides from the extracellular space to the MHC class I antigen processing pathway of antigen presentation cells. Grp94 and Hsp90 share high sequence identity and apparently identical adenosine nucleotide dependent modes of regulation, although previous data suggests that Hsp90 and Grp94 may differ in their nucleotide binding properties. The N-terminal domain of eukaryotic Hsp90 proteins contains a conserved adenosine nucleotide binding pocket which also serves as the binding site for the Hsp90 inhibitors geldanamycin and radicicol. However, the molecular basis for adenosine nucleotide-dependent regulation of Grp94 remains unclear. Data supports a ligand dependent regulation of Grp94 function, and suggests a model whereby Grp94 function is regulated through a ligand -dependent conversion of Grp94 from an inactive to an active conformation. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |