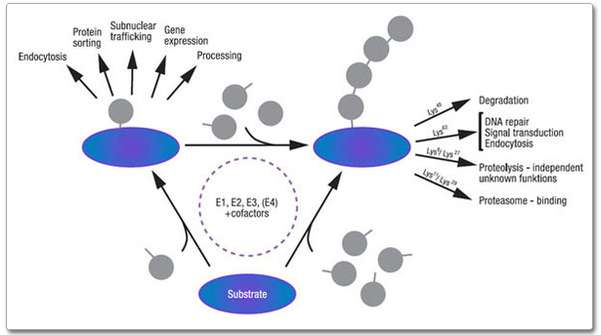
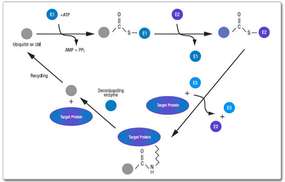
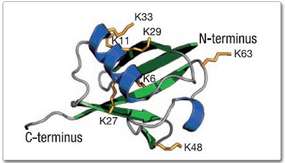
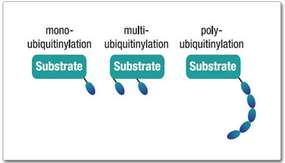
Products
Technology Platforms | Markets | Applied Science | Browse Products | Quick Links |
Science Center
Scientific Literature | TechNotes | Interesting | Social Media |