-
New formulation enhances gelling over a larger concentration range.
Product Details
| Alternative Name: | COL1 |
| |
| MW: | 235kDa, 215kDa, 130kDa, 115kDa |
| |
| Source: | Isolated from rat tail tendons. |
| |
| Concentration: | 5mg/ml |
| |
| Formulation: | Sterile liquid. In 0.02N acetic acid. |
| |
| Purity: | ≥90% (SDS-PAGE) |
| |
| Endotoxin Content: | <100 EU/mg purified protein (LAL test) |
| |
| Appearance: | Hazy viscous liquid. |
| |
| Activity: | Tested in cell proliferation assay – Increased attachment of cells on collagen coated coverslips. |
| |
| Application Notes: | Can be used for preparation of collagen gels, thin layer coating of surfaces (culture plates, slides, coverslips). |
| |
| Shipping: | Blue Ice Not Frozen |
| |
| Short Term Storage: | +4°C |
| |
| Long Term Storage: | +4°C |
| |
| Handling: | Keep sterile. Do not freeze. |
| |
| Scientific Background: | Collagen is the main component in connective tissue and helps to provide support for tissues. It is made up of several classes, with Type 1 collagen being the most common. Type 1 collagen has a herterotrimeric triple helical structure made up of two alpha-1(I) and one alpha-2(I) chains that form into elongated fibrils which are extremely strong. These fibrils can be found in skin, tendons, ligaments, and other connective tissues. Type 1 collagen has been shown to be useful as a substrate that promotes cell growth and proliferation. Under acidic conditions the protein is soluble, however by raising the temperature and pH the solution forms into a solid gel that can be useful for cellular studies. It can also be dried to form a thin layer on solid surfaces such as plates, slides, or coverslips to aid in cell attachment. |
| |
| Protocol: | Firm Gelling Procedure
(Note: The ideal concentration for gelling is 1 - 5mg/ml. The end user will need to determine the appropriate concentration for their specific purpose).
Ammonium Hydroxide Gas Method
-
Dilute 5mg/ml collagen to the desired concentration using sterile conditions. (Note: Lower concentrations will have reduced rigidity).
-
Add enough collagen to cover the surface.
-
Saturate a cotton ball or piece of filter paper with concentrated ammonium hydroxide and make a closed vapor chamber.
-
Heat to 37°C until gel forms.
-
Remove the ammonium hydroxide.
-
Soak the gel surface in PBS for 1 hour.
-
Wash several times PBS.
-
Store the gel in PBS at +4°C until use.
-
Equilibrate the gel in the desired media for 30 minutes prior to use.
NaOH Method (For 10ml)
-
Sterilely add 1- 7ml of 5mg/ml collagen to a tube depending on desired final concentration. (Note: Lower concentrations will have reduced rigidity).
-
Add 1ml of sterile 10X PBS.
-
Add 1 ml of sterile 1N NaOH.
-
Bring final volume to 10ml with sterile dH2O
-
Cover the desired surface with a layer of the collagen and place at 37°C until the gel has solidified. Gel will only form if the pH is ≥7.0.
-
Store in PBS at +4°C until use.
-
Equilibrate the gel in the desired media for 30 minutes prior to use.
|
| |
| Regulatory Status: | RUO - Research Use Only |
| |
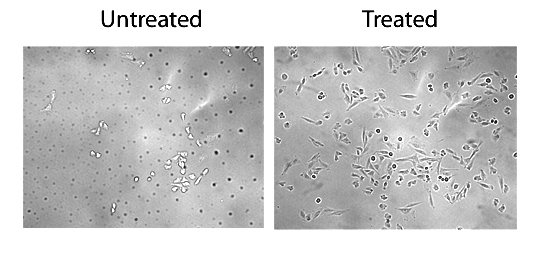
Glass coverslips were coated with or without collagen in 60% ethanol and allowed to dry overnight. CHO cells were plated at onto the coverslips and grown for two days at 37oC with 5% CO2. Cells were fixed in 10% buffered formalin. Coverslips were washed in PBS, mounted upside down onto microscope slides, and imaged with a 20X objective.
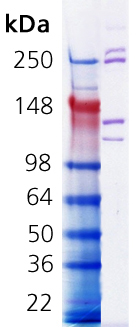
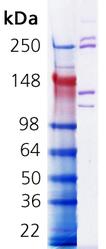
Coomassie stained SDS-PAGE. Lane 1, Molecular weight marker. Lane 2, 1.0µg Collagen I, rat tail.
Please mouse over
Product Literature References
Modeling Gas Plasma-Tissue Interactions in 3D Collagen-Based Hydrogel Cancer Cell Cultures: L. Miebach, et al.; Bioengineering (Basel)
10, 367 (2023),
Abstract;
A TNF receptor 2 agonist ameliorates neuropathology and improves cognition in an Alzheimer’s disease mouse model: N.O. Casan, et al.; PNAS
119, e2201137119 (2022),
Abstract;
Axon guidance receptor ROBO3 modulates subtype identity and prognosis via AXL-associated inflammatory network in pancreatic cancer: N. Krebs, et al.; JCI Insight
7, e154475 (2022),
Abstract;
Cooperative interaction between ERα and the EMT-inducer ZEB1 reprograms breast cancer cells for bone metastasis: N.M. Ghahhari, et al.; Nat. Commun.
13, 2104 (2022),
Abstract;
Efficacy of probiotic Streptococcus thermophilus in counteracting TGF-β1-induced fibrotic response in normal human dermal fibroblasts: F. Lombardi, et al.; J. Inflamm.
19, 27 (2022),
Abstract;
SIRT1-mediated deacetylation of FOXO3a transcription factor supports pro-angiogenic activity of interferon-deficient tumor-associated neutrophils: S. Bordbari, et al.; Int. J. Cancer
150, 1198 (2022),
Abstract;
Integrin β3 targeting biomaterial preferentially promotes secretion of bFGF and viability of iPSC-derived vascular smooth muscle cells: B. C. Dash, et al.; Biomater. Sci.
9, 5319 (2021),
Abstract;
Low nanogel stiffness favors nanogel transcytosis across an in vitro blood-brain barrier: L. Ribovski, et al.; Nanomedicine
34, 102377 (2021),
Abstract;
Low nanogel stiffness favors nanogel transcytosis across an in vitro blood–brain barrier: L. Ribovski, et al.; Nanomedicine
34, 102377 (2021),
Abstract;
A new perfusion culture method with a self-organized capillary network: K. Sugihara, et al.; PLoS One
15, e0240552 (2020),
Abstract;
Full Text
Induced pluripotent stem cell-derived smooth muscle cells increase angiogenesis and accelerate diabetic wound healing: J. Gorecka, et al.; Regen. Med.
15, 1277 (2020),
Abstract;
Full Text
A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy: E. De Jong, et al.; J. Control. Release
289, 14 (2018),
Abstract;
Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts: M. Cutolo, et al.; Arthritis Res. Ther.
20, 77 (2018),
Abstract;
Full Text
General Literature References
Reconstituted rat-tail collagen used as substrate for tissue cultures on coverslips in maximow slides and roller tube: M.B. Bornstein, et al.; Lab. Invest. 134, (1958),