Due to its flexibility, Western blotting can be used for a wide range of applications within molecular biology and biochemistry, including detection of post-translational modifications and verification of protein cloning. Immobilized membrane-bound proteins are generally detected using secondary antibodies that are conjugated to fluorescent molecules (fluorophores) or an enzyme such as alkaline phosphatase (AP) or horseradish peroxidase (HRP). When the enzyme substrate is added, either a colored precipitate (colorimetric detection) or a chemiluminescent or fluorescent product is formed and the light signal is captured. Using the size and color intensity of the protein band, a semi-quantitative estimation of protein can be derived. The new
Chromogenic Western Blot Kit allows for quick, visual reading of Western blots in unpurified cell extract. The kit can be used to identify primary mouse antibodies on a Western blot using immunodetection. It contains a complete reagent set, with optimized ready-to-use or ready-to-dilute reagents.
Chemiluminescence Detection
Chemiluminescent detection systems take advantage of energy released in reporter enzyme-substrate reactions in the form of light to be the recorded signal. The two commonly used enzyme reporters are HRP and AP. The choice of substrate for chemiluminescent Western blotting is determined by which reported enzyme is chosen. If utilizing HRP, luminol-based reagents are selected. HRP in a peroxide buffer oxidizes luminol to its excited state product, 3-aminophthalate, which emits a light signal at 425nm. When utilizing AP, 1, 2-dioxetane-based reagents are the adjacent AP substrates. AP dephosphorylates 1, 2-dioxetane-based substrates to yield a dioxetane phenolate anion that emits a light signal at 466nm. These light signals are then captured on X-ray film or charge-coupled device (CCD) cameras as the light signal decays to the ground state. Chemiluminescent systems are easily adapted to traditional Western blotting protocols due to the use of enzyme-conjugated antibodies to activate the light signal. Once an end-user has optimized the correct concentration of enzyme-conjugated antibody, a transient chemiluminescent signal is produced that decays quickly upon exhaustion of substrate. Both methods are compatible on nitrocellulose or PVDF membrane blots.
One advantage of chemiluminescent Western blot systems is the sensitivity. HRP-substrate reactions can detect down to 1 to 3 pg and AP-substrate reactions can detect as little as 10 pg of protein. Given the short lived nature of chemiluminescent signals, enhancer chemiluminescent substrates (ECL) have been developed which enable increased signal duration, intensity, and sensitivity. High sensitivity can, however, be disadvantageous in samples that present high background and the end-user must still examine how the affinity of the protein, primary antibody, secondary antibody, and reporter enzyme substrate will vary in performance from one sample to another and make appropriate optimizations.
Chemiluminescent-based methods offer much higher sensitivity relative to colorimetricbased methods that detect proteins within the 100pg-500pg range or fluorescentbased methods which are limited to the nanogram (10-9) range. With that being said, higher sensitivity can be disadvantageous in samples that present high background and the end-user must still examine how the affinity of the protein, primary antibody, secondary antibody, and reporter enzymesubstrate will vary in performance from one sample to another and make appropriate optimizations.
Another advantage of chemiluminescent systems is the speed at which a protein can be detected. Signals captured with film or CCD imaging cameras offer short exposure times that are 30 seconds to 5 minutes depending on if you use HRP or AP, respectively. Films are limited to one-time use and must be developed before one can know whether the exposure time was adequate or not, which can lead to frustrating trial and error. Modern CCD imaging systems allow for high dynamic range and a variety of different exposures, thus enabling the end user to analyze a broad spectrum of protein concentrations within a given sample. Image processing algorithms not only allow for clear specific bands and low background, but also easy semi-quantitative analysis of the results.
Chromogenic Detection
Unlike chemiluminescent or fluorescent blotting applications, chromogenic substrates do not require special imaging equipment for visualization of the assay results. Chromogenic detection is an economical and far less labor intensive method for the detection of target protein during a Western blot protocol. Much like chemiluminescent detection, chromogenic detection uses a secondary antibody conjugated to a reporter enzyme that is either HRP or AP. Where chromogenic detection departs from chemiluminescent detection is in the way detectable signal is produced. Instead of reacting with a chemiluminescent substrate, reporter enzymes will react with a soluble, chromogenic substrate to produce a colored, insoluble product which is the detectable signal. This signal precipitates directly onto the blotting membrane to produce colored bands that can be seen by the eye and inform the end-user of the presence or absence of a protein of interest. Similar to film development, the blot is incubated until the desired amount of development is achieved which requires some trial and error to optimize signal intensity. Initially, an end-user must be weary of prolonged incubation as it can increase background signal which could obscure the detectable signal. However, the enzymatic reaction can be visually monitored so the reaction can be stopped with relative ease. This feature makes chromogenic detection a medium-sensitivity technique that is unsuitable for samples with low quantities of the protein of interest.
For chromogenic HRP systems, the typical chromogen substrates are 3,3’,5,5’-tetramethylbenzidine (TMB), chloro-1-napthol (4CN) and 3, 3’-diaminobenzidine (DAB). TMB reacts with HRP in a peroxide buffer to produce a water-soluble blue product that is suited for applications that require a large signal to noise ratio. 4CN produces a distinct blue-purple product that is useful for double staining despite the lack of sensitivity or stability afforded by TMB and DAB. DAB yields a brown colored precipitate. The color produced by DAB is intensified by adding metals (nickel, copper, silver, and cobalt) that produce complexes that result in darker colored product and thus, enhance the sensitivity in staining.
For chromogenic AP systems, the typical chromogen substrates are nitro blue tetrazolium (NBT) or 5-bromo-4-chloro-indolyl phosphate (BCIP). NBT is a heterocyclic tetrazolium salt that reduces to yield NBT-formazan, an intensely colored, water-insoluble product that is stable. Therefore, the reaction product will not fade as compared to chromogenic HRP enzyme-substrate products which can fade with exposure to light. NBT is hydrolyzed by AP and produces a blue-purple precipitate that is deposited on nitrocellulose membranes. Unlike chromogenic HRP systems, AP reactions can be inactivated with acidic solutions allowing for multiple probing of the same membrane with alternatively colored antibody probes. AP systems compared to HRP systems will not require such intense chemical stripping that can make the membrane difficult to re-probe for other targets. Ideally though, chromogenic Western blots with AP use a combination of NBT and BCIP that result in an intense, black-purple precipitate which provides a greater substrate sensitivity. NBT/BCIP substrate combinations with AP produce sharp bands with little background staining which is ideal for Western blot application.
The
Chromogenic Western Blot Kit takes advantage of the resource and time efficiency
and simplicity of a chromogenic format and the enhanced sensitivity and flexibility of an AP system to provide end-users with highly sensitive and flexible immunodetection through an already optimized, ready-to-use NBT/BCIP solution that is used with antimouse secondary antibody conjugated to reporter enzyme. The kit comes with antibody blocker/diluents and wash buffers that are compatible with mouse primary antibodies. During product validation, we have validated assay times of 2 hours with NBT/BCIP exposures times of as little as a minute with low abundance protein targets in Jurkat cell lysates (Figure 1 and 2).
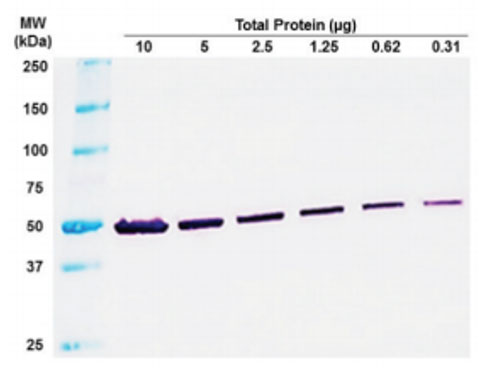
Figure 1: Visual Reading for Rapid Results. Total cell lysate from Jurkat cells. Exposure to NBT/BCIP for 1 minute.
|
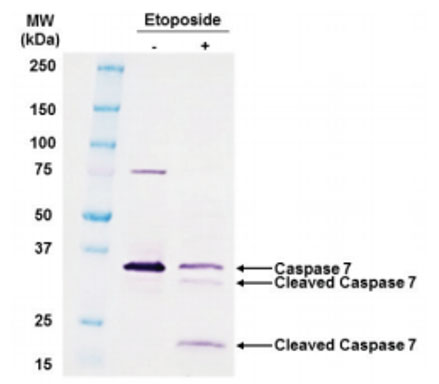
Figure 2: Detect Difficult Proteins. Jurkat cells treated with 12.5 μM etoposide for 18 hours. Loaded 12 μg lysate per well.
|
Furthermore, this kit utilizes an AP system that affords end-users the opportunity to multiplex for multiple targets of interest in a single sample so long as the enduser selects a different colored substrate (Figure 3).
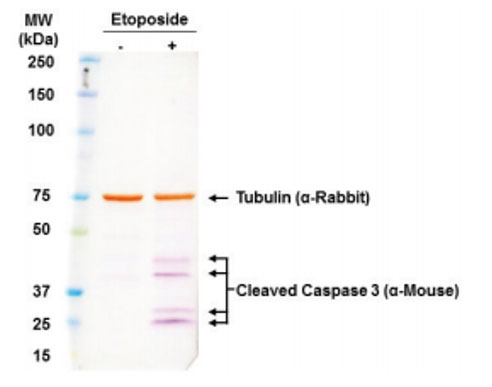
Figure 3: Adaptable to Multiplex Analysis. PANC-1 cells treated with Etoposide. 20 μg of total cell lysate loaded. Tubulin labeled with DAB. Caspase labeled with NBT/BCIP.
|
Fluorescent Detection
Much like enzyme detection systems, fluorescent detection still uses antigen-antibody complex’s to detect specific proteins that have been immobilized on a blot membrane after separation by gel electrophoresis. Fluorescent Western blot detection differs from chemiluminescent detection in the use of fluorochrome-coupled secondary antibodies. The resulting signal is detected with a fluorescence imaging system similar to epifluorescence microscopy. The fluorochrome bound to the antibody is excited with a light source, and the emitted fluorescent signal is detected with the use of wavelengthspecific filters and digital camera systems.
A key feature that separates fluorescent detection from enzyme detection systems is that it is more quantitative. Chromogenic or chemiluminescent detection provides a researcher with a semi-quantitative method because the end-user is able to determine the presence or absence of their protein of interest in their samples and through comparison between samples you can infer the expression level of a protein. But with fluorescent detection, the amount of fluorescence produced by a signal is directly proportional to the amount of protein present. However, as in other methods, the end-user still needs to optimize the signal-to-background ratio. Initially, important fluorophore characteristics need to be considered in both fluorophore selection and signal optimization such as the efficiency of photon emission after absorption of light-source photon (quantum yield) and how well a fluorophore absorbs light at a specific wavelength (extinction coefficient). Collectively, these will indicate the relative brightness of the fluorophore.
Fluorophores with higher quantum yields and extinction coefficient are generally brighter. In situations where the end-user is expecting lower-abundance targets in the sample, high initial brightness could be helpful for detection. The difference in maximum excitation and emission wavelengths of a fluorophore (stokes shift) should be considered for signal optimization as well. Fluorophores with larger stokes shifts can minimize overlap between excitation and emission wavelengths while increasing the signal intensity. Additionally, the degree of labeling of the secondary antibody with fluorophore may need to be considered. Too little fluorescent labeling and the fluorescence intensity will generate a weak signal. Low signal can also be due to low antigen or excessive washing of the blotting membrane, even if a secondary antibody is used to amplify the signal. Too much fluorescent labeling and the signal will be weak because the detection reagent will be inactivated and the signal will quench due to Forster Resonance Energy Transfer (FRET). To mitigate this, far-red and infrared dye conjugates such as Alexa Fluor 680 and 790 are sometimes used because they offer low quenching, low background fluorescence with high extinction coefficient producing a sensitive, robust signal. Last but not least, the excitation and emission spectra of each fluorophore should be considered in multiplex Westerns to avoid overlap.
Fluorescent Western blot affords several advantages over other methods. For example, the ability to easily multiplex or use several, different colored fluorophores to probe for and detect multiple proteins on the same blot simultaneously. The different colored fluorophores are to differentiate between target proteins. Unlike enzyme-based methods, there is no need to chemically strip and re-probe the blot when looking at multiple targets. Additionally, the end-user does not need to consider the impact of substrate incubation time or film exposure on detecting a clear signal. However, the end-user does need to consider the potential for cross-reactivity and use primary antibodies from different host species and secondary antibodies that are cross-absorbed against other species. Another advantage comes from the stability of fluorophores. Signals are stable for weeks to months, allowing blots to be stored for re-imaging at a later time point without significant loss of signal.
|
Fluorescent Detection |
Chemiluminescent Detection |
Chromogenic Detection |
| Substrates |
Fluorescent dyes and conjugated secondary antibodies |
AP and HRP substrates |
Color detection reagents |
| Detection |
Imaging instruments with appropriate filters or lasers |
Film and developer |
No instrumentation required |
| Signal Duration |
Weeks |
Hours |
Months |
| Considerations |
Can simultaneously detect multiple proteins on the same blot |
Excellent sensitivity with a wide variety of substrates available |
Direct visualization with no imaging or film processing instrumentation required |
Table 1. Western Blot Detection Techniques.
Enzo Life Sciences offers thousands of
antibodies against peptides, whole or partial proteins, and modified peptides or proteins. Backed by thousands of peer-reviewed citations, our
Worry-free Antibody Trial Program, and expert technical support, you can count on Enzo antibodies to deliver reliable, consistent, and accurate data. Feel free to check out our
Successful Research Tips or our
10 Tips for Successful Westerns. Additionally, contact our
Technical Support Team for further assistance.