Development of a single drug, whether it is a new chemical entity, biotherapeutic, or genetic/cellular therapy, requires significant investment of resources. Each step of the process from early discovery to production and delivery must be fully explored, characterized, and understood to prevent issues resulting from cell stress, cell death, protein aggregation, and factors affecting reliable manufacturing of the drug. Whether you work in drug discovery, or upstream or downstream bioprocessing, Enzo offers a range of products to help you maintain cell line viability, optimize and monitor product integrity, and maximize yield.
Protein A ELISA Kit
Protein A is a bacterial protein produced in
Staphylococcus aureus which binds to IgGs with fidelity. Therefore, this recombinant protein has found wide use in affinity purification of antibodies and in commonly used immunodetection and visualization techniques. Protein A participates in a number of biological functions including toxic and carcinogenic activity, thus necessitating the removal of potential Protein A contaminants from antibody preparations for therapeutic use.
Protein A is commonly used by the industry to purify monoclonal antibodies (mAbs) from harvest cell culture supernatant
1. Protein A affinity chromatography has been shown to be highly selective for mAbs, resulting in a purity greater than 95% starting from complex samples. It became the method of choice by achieving high purity and yield in a single unit operation, simplifying manufacturing operations, and reducing the time and effort required to develop the purification process.
Up to 100% Recovery of Natural and Recombinant Proteins
 Figure 1.
Figure 1. Assay recognition of different Protein A constructs, post boiling. Resulting concentrations were interpolated from kit standard curve. Percent recovery calculated by dividing observed concentration by expected concentration. A: Natural Protein A from
S. aureus (Competitor M), n=9; B: Recombinant Protein A from
E. coli (Competitor R), n=9; C: Recombinant Cys-Protein A from
E. coli (Competitor G), n=12; and D: Recombinant alkaline-resistant Protein A variant from
E. coli (Competitor S), n=12; graphical data represents statistical mean ± 1 standard deviation.
In a typical mAb purification process, the protein-rich supernatant is harvested from hybridoma cells in culture. Then, during Protein A affinity chromatography, antibodies are captured at a neutral pH and eluted at an acidic pH. After the inactivation of foreign viruses, cation exchange chromatography is performed to remove host cell proteins, mAb aggregates, and antibody fragments. This is followed by anion exchange chromatography, which removes DNA, leached Protein A, and other contaminants. After these filtrations are complete, the biologic is ready for use.
Enzo’s
Protein A ELISA Kit is the most sensitive ELISA kit on the market, allowing for the detection of 9.0 pg/mL of Protein A residuals in purified humanized mAb preparations. The kit recognizes both native and recombinant Protein A, ensuring accurate and reliable results. Additionally, the kit can be run in < 3 hours for up to 37 samples in duplicate, and is a cost-effective solution compared to other technologies.
Host Cell Protein ELISAs
Host Cell Proteins (HCPs) are a residual contaminant that can appear in the production of a biologic. HCPs are produced by organisms as a side product to the intended protein. While some are necessary for natural cellular functions, other HCPs are unnecessary and are merely carried through the process. Even though some may be completely harmless, it is generally undesirable to have HCPs in the final product. Every year, considerable effort is expended in the industry to remove HCPs from biologic mixes. This is complicated by the tendency of HCPs to be present in very small quantities (usually on the ng/mL scale), making it additionally important to quantify HCP residuals in the production of biologics.
Enzo Life Sciences offers two different HCP ELISAs: a kit to quantify HCPs in biologics produced in
E. coli and another to quantify HCPs in Chinese Hamster Ovary (CHO)-based processes. The assays are high-throughput with results in three hours or less, and sensitive with the ability to detect HCPs from 3 to 810 ng/mL. Our dynamic range allows the end user to detect low and high contamination levels, reducing the need for repeated tests due to out-of-range samples.
PROTEOSTAT® Protein Aggregation Assay
PROTEOSTAT® Protein Aggregation Assay provides a simple mix-and-read assay format for monitoring peptide and protein aggregation in solution. This assay, which can be used in a flow cytometry or fluorescence application, is useful for defining optimal storage conditions and formulations for proteins, for screening compounds that promote or inhibit protein aggregation, and potentially for measuring molecular chaperone activity in a sensitive manner. The assay can be employed to streamline protein processing and formulation optimization procedures. Relative to conventional protein aggregation detection dyes, such as Thioflavin T, Enzo’s PROTEOSTAT® detection reagent can detect visible to subvisible aggregates from a broader range of proteins, yields a much brighter signal, provides at least two orders of magnitude linear dynamic range, and offers superior performance across a broad range of pH values (4-10) and buffer compositions. Sensitivity for this assay is in the sub-micromolar range and as little as 1-5% protein aggregate is detectable in a concentrated protein solution. The assay is capable of providing quantitative analysis of protein aggregation in a robust and high-throughput fashion (Z’ factor score >0.5). Lyophilized native and aggregated protein are provided as negative and positive controls for monitoring changes in protein aggregation status.
Fluorescence Gain Upon Aggregation of Lysozyme (%)
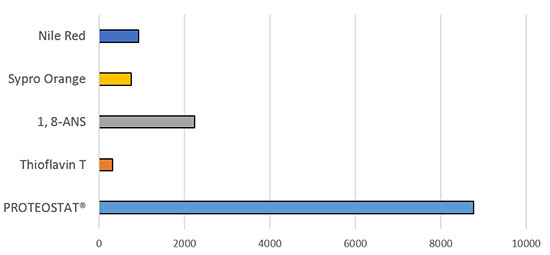 Figure 3.
Figure 3. Enzo’s PROTEOSTAT® Protein Aggregation Assay offers superior detection of subvisible protein aggregates compared to conventional dyes.
Conclusion
Enzo’s catalog of widely cited and thoroughly validated ELISAs and antibodies includes bioprocess-focused kits for detection of Protein A and HCP residuals, with a full line of PROTEOSTAT® assays for contamination monitoring and process optimization. This offering is supported by
over 300 ELISAs and more than
3,000 antibodies to enhance your discovery and manufacturing workflow.
Please check out our
Bioprocess Optimization platform for more information or contact our
Technical Support Team for further assistance.











