Lipid peroxidation is a well-established mechanism of cellular injury in both plants and animals, and is used as an indicator of oxidative stress in cells and tissues. Lipid peroxides, derived from polyunsaturated fatty acids, are unstable and decompose to form a complex series of compounds. These include reactive aldehydes, of which the most abundant is malondialdehyde (MDA). Therefore, measurement of malondialdehyde is widely used as an indicator of lipid peroxidation. Increased levels of lipid peroxidation products have been associated with a variety of chronic diseases in both humans and model systems. MDA reacts readily with amino groups on proteins and other biomolecules to form a variety of adducts, including cross-linked products. MDA also forms adducts with DNA bases that are mutagenic and possibly carcinogenic. DNA-protein cross-links are another result of the reaction between DNA and MDA . The TBARS method is commonly used to measure MDA in biological samples. However, this reaction is relatively nonspecific; both free and protein-bound MDA can react. The BML-AK171 method is designed to assay free MDA or, after a hydrolysis step, total MDA (i.e., free and protein-bound Schiff base conjugates). The assay conditions serve to minimize interference from other lipid peroxidation products, such as 4-hydroxyalkenals.
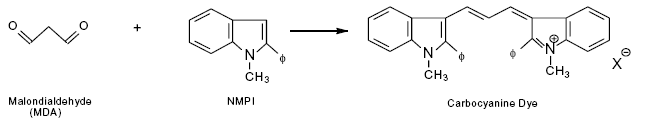
Figure 1: N-methyl-2-phenylindole (NMPI) reacts with malondialdehyde to form an intensely colored carbocyanine dye with a maximum absorption at 586 nm.
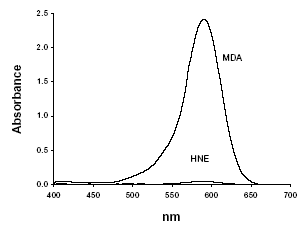
Figure 2: Absorption spectra obtained from the reaction of NMPI with MDA (21 µM) or HNE (19 µM) in the presence of hydrochloric acid.
Please mouse over
Product Details
| Applications: | Colorimetric detection
|
| |
| Use/Stability: | Store stock reagents at 4°C. Do not allowed the capped reagent bottles to sit at room temperature for long periods of time. Unopened reagents are stable until the indicated expiration date. |
| |
| Handling: | Do not freeze. |
| |
| Shipping: | Blue Ice Not Frozen - Dangerous Good |
| |
| Long Term Storage: | +4°C |
| |
| Contents: |
Reagent R1 (Prod. No. BML-KI527) (3x18ml;N-methyl-2-phenylindole, in acetonitrile)
Reagent R2 (Prod. No. BML-KI606) (1x16.5ml; Conc. Hydrochloric acid)
MDA Standard (Prod. No. BML-KI529) (1ml; 1,1,3,3-tetramethoxypropane (TMOP) in Tris-hydrochloric acid)
BHT (Prod. No. BML-KI530) (2ml; Butylated hydroxytoluene in acetonitrile)
Probucol (Prod. No. BML-KI531) (1.1ml; Probucol in methanol)
Methanol (Prod. No. BML-KI532) (30ml) |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
Resveratrol and/or exercise training counteract aging-associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function: M.H. Muhammad, et al.; J. Physiol. Sci.
68, 681 (2018),
Abstract;
The clinically used PARP inhibitor olaparib improves organ function, suppresses inflammatory responses and accelerates wound healing in a murine model of third-degree burn injury: A. Ahmad, et al.; Br. J. Pharmacol.
175, 232 (2018),
Abstract;
Full Text
AP39, A Mitochondrially Targeted Hydrogen Sulfide Donor, Exerts Protective Effects in Renal Epithelial Cells Subjected to Oxidative Stress in Vitro and in Acute Renal Injury in Vivo: A. Ahmad, et al.; Shock
45, 88 (2016),
Abstract;
Full Text
Both the H2S biosynthesis inhibitor aminooxyacetic acid and the mitochondrially targeted H2S donor AP39 exert protective effects in a mouse model of burn injury: A. Ahmad, et al.; Pharmacol. Res.
113, 348 (2016),
Abstract;
Delayed Treatment with Sodium Hydrosulfide Improves Regional Blood Flow and Alleviates Cecal Ligation and Puncture (CLP)-Induced Septic Shock: A. Ahmad, et al.; Shock
46, 183 (2016),
Abstract;
Full Text
Effect of endotoxemia in mice genetically deficient in cystathionine-γ-lyase, cystathionine-β-synthase or 3-mercaptopyruvate sulfurtransferase: A. Ahmad, et al.; Int. J. Mol. Med.
38, 1683 (2016),
Abstract;
Full Text
Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice: M. Kobori, et al.; J. Funct. Foods 15, 551 (2015), Application(s): Assay,
Anti-nephrotoxic activity of some medicinal plants from tribal rich pockets of Odisha: S. Mishra, et al.; Pharmacognosy Res.
6, 210 (2014),
Abstract;
Full Text
Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture: C. Coletta, et al.; Crit. Care
18, 511 (2014),
Abstract;
Full Text
General Literature References
Induction of apoptosis by arachidonic acid in human retinoblastoma Y79 cells: involvement of oxidative stress: R. Vento et al.; Exp. Eye Res.
70, 503 (2000),
Abstract;
Analysis of DNA-protein crosslinking activity of malondialdehyde in vitro: V. Voitkun et al.; Mutat. Res.
424, 97 (1999),
Abstract;
Chemistry and biology of DNA damage by malondialdehyde: L.J. Marnett et al.; IARC Sci. Publ. 17 (1999),
Abstract;
Lipid peroxidation-DNA damage by malondialdehyde: L.J. Marnett et al.; Mutat. Res.
424, 83 (1999),
Abstract;
Oxidative stress in Huntington’s disease: S.E. Browne et al.; Brain Pathol.
9, 147 (1999),
Abstract;
Solution structure of an oligodeoxynucleotide containing the malondialdehyde deoxyguanosine adduct N2-(3-oxo-1-propenyl)-dG (ring-opened M1G) positioned in a (CpG)3 frameshift hotspot of the Salmonella typhimurium hisD3052 gene: H. Mao et al.; Biochemistry
38, 13491 (1999),
Abstract;
Formation of liver microsomal MDA-protein adducts in mice with chronic dietary iron overload: L.G. Valeria et al.; Toxicol. Lett.
98, 31 (1998),
Abstract;
Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Mechanistic aspects of the colorimetric assay of lipid peroxidation: I. Erdelmeier et al.; Chem. Res. Toxicol.
11, 1184 (1998),
Abstract;
US Patent No. US5726063: D. Gérard-Monnier et al.; (1998),
D. Gérard-Monnier et al.; Chem. Res. Toxicol. 11, 10 1176 (1997),
Assay of aldehydes from lipid peroxidation: gas chromatography-mass spectrometry compared to thiobarbituric acid: J. Liu et al.; Anal. Biochem.
245, 161 (1997),
Abstract;
Association between reactive oxygen species and disease activity in chronic hepatitis C: N. De Maria et al.; Free Radic. Biol. Med. 21, 291 (1996),
N.A. Botsoglou ; J. Agric. Food. Chem. 42, 1931 (1994),
NCCLS Tentative Guideline; 2nd ed. National Committee for Clinical Laboratory EP5-T2, (1992),
Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes: H. Esterbauer et al.; Free Radic Biol Med
11, 81 (1991),
Abstract;
Free and bound malondialdehyde measured as thiobarbituric acid adduct by HPLC in serum and plasma: M.A. Carbonneaou et al.; Clin. Chem.
37, 1423 (1991),
Abstract;
Interaction of malondialdehyde-modified bovine serum albumin and mouse peritoneal macrophages: M. Beppu et al.; Chem Pharm Bull (Tokyo)
36, 4519 (1988),
Abstract;
Determination of malondialdehyde by ion-pairing high-performance liquid chromatography: A.W. Bull et al.; Anal. Biochem.
149, 284 (1985),
Abstract;