Product Details
| Alternative Name: | Heat shock protein 90 |
| |
| Clone: | 2D11B9 |
| |
| Host: | Mouse |
| |
| Isotype: | IgG1 |
| |
| Immunogen: | Recombinant human Hsp90. |
| |
| UniProt ID: | P07900 (HSP90α), P08238 (HSP90β) |
| |
| Source: | Purified from ascites. |
| |
| Species reactivity: | Human, Mouse, Rat
Dog, Drosophila, Fish, Guinea pig, Hamster, Horse, Monkey, Porcine, Sheep
|
| |
| Applications: | WB
|
| |
| Recommended Dilutions/Conditions: | Western Blot (1µg/ml, colorimetric)
Suggested dilutions/conditions may not be available for all applications.
Optimal conditions must be determined individually for each application. |
| |
| Application Notes: | Detects a band of ~92kDa by Western blot. |
| |
| Purity Detail: | Protein G affinity purified. |
| |
| Formulation: | Liquid. In PBS containing 50% glycerol and 0.09% sodium azide. |
| |
| Handling: | Avoid freeze/thaw cycles. |
| |
| Shipping: | Blue Ice |
| |
| Long Term Storage: | -20°C |
| |
| Scientific Background: | The 90kDa molecular chaperone family includes 90kDa heat shock protein Hsp90 and 94kDa glucose-regulated protein grp94, both major molecular chaperones of the cytosol and the endoplasmic reticulum. Mammalian cells contain isoforms Hsp90α and Hsp90β, encoded by separate genes. The amino acid sequences of human and yeast Hsp90α are 85% and 90% homologous to those of Hsp90β, respectively. All known members of the Hsp90 protein family are highly conserved, especially in the N-terminal and C-terminal regions containing independent chaperone sites with different substrate specificity. These ubiquitous and highly conserved proteins account for 1-2% of all cellular proteins in most cells. Hsp90 functions as part of the cell’s powerful network of chaperones to fight the deleterious consequences of protein unfolding caused by non-physiological conditions. In the absence of stress, however, Hsp90 provides a necessary component of such fundamental cellular processes as hormone signalling and cell cycle control. In this context, researchers identified key regulatory proteins as substrates of Hsp90, including steroid receptors, cell cycle kinases involved in signal transduction, and p53. Hsp90 may act as a capacitor for morphological evolution by buffering widespread variation, potentially affecting morphogenic pathways. When temperature and other stress factors compromise Drosophilia Hsp90 buffering, cryptic variant expression occurs, and selection can lead to the continued expression of these traits, even after Hsp90 function is restored. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
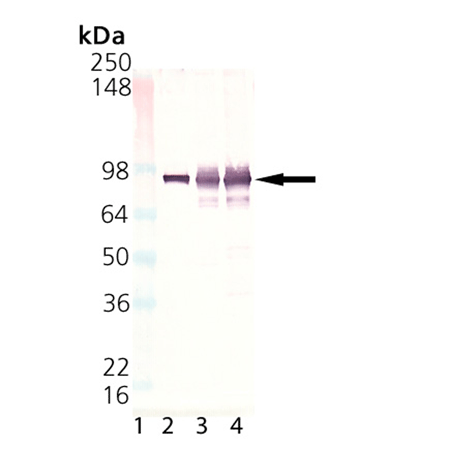
Western Blot Analysis of HSP90, mAb (2D11B9) (Prod. No. ADI-SPA-831). Lane 1: MWM; Lane 2: HSP90 (human), (native) (Prod. No. ADI-SPP-770); Lane 3: HeLa, (cell lysate) (Prod. No. ADI-LYC-HL100); and Lane 4: HeLa (heat shocked), (cell lysate) (Prod. No. ADI-LYC-HL101).
Please mouse over
Product Literature References
The Step of Incorporation of Bacillus coagulans GBI-30 6086 Into “requeijão cremoso” Processed Cheese Does Not Affect Metabolic Homeostasis of Rats: M.B. Soares, et al.; Front. Microbiol.
10, 2332 (2019),
Abstract;
Full Text
Fluorescent ligand fishing combination with in-situ imaging and characterizing to screen Hsp 90 inhibitors from Curcuma longa L. based on InP/ZnS quantum dots embedded mesoporous nanoparticles: Y. Hu, et al.; Talanta
178, 258 (2018),
Abstract;
Impact of Trans‐Fats on Heat‐Shock Protein Expression and the Gut Microbiota Profile of Mice: G.C.B.C. Carvalho, et al.; J. Food Sci.
83, 489 (2018),
Abstract;
Limonene reduces hyperalgesia induced by gp120 and cytokines by modulation of IL-1 β and protein expression in spinal cord of mice: A.C. Picinelli, et al.; Life Sci.
174, 28 (2017),
Abstract;
Modulatory effects of arginine, glutamine and branched-chain amino acids on heat shock proteins, immunity and antioxidant response in exercised rats: C.S. Moura, et al.; Food Funct.
8, 3228 (2017),
Abstract;
Novel HSP90 inhibitors effectively target functions of thyroid cancer stem cell preventing migration and invasion: P.T. White, et al.; Surgery
159, 142 (2015),
Application(s): Western blot,
Abstract;
Salternamide A Suppresses Hypoxia-Induced Accumulation of HIF-1α and Induces Apoptosis in Human Colorectal Cancer Cells: D.H. Bach, et al.; Mar. Drugs
13, 6976 (2015),
Application(s): Immunoblotting of human colorectal cancer cells,
Abstract;
Full Text
General Literature References
The charged region of Hsp90 modulates the function of the N-terminal domain: T. Scheibel, et al.; PNAS USA
96, 1297 (1999),
Abstract;
Hsp90 as a capacitor for morphological evolution: S.L. Rutherford & S. Lindquist; Nature
396, 336 (1998),
Abstract;
Oligomeric forms of the 90-kDa heat shock protein: T. Nemoto & N. Sato; Biochem. J.
330, 989 (1998),
Abstract;
The Hsp90 complex--a super-chaperone machine as a novel drug target: T. Scheibel & J. Buchner; Biochem. Pharmacol.
56, 675 (1998),
Abstract;
Guidebook to Chaperones: T. Scheibel & J. Buchner; Ed. Gething, M.J. Oxford Univ. Press 147 (1997), Book,
Related Products