COVID-19 Molecular Testing
AMPIPROBE® SARS-CoV-2 Test System

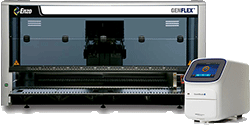
The AMPIPROBE® SARS-CoV-2 Test System, which is comprised of the AMPIXTRACT SARS-CoV-2 Extraction Kit, the AMPIPROBE® SARS-CoV-2 Assay kit and the AMPIPROBE SARS-CoV-2 Controls Kit, is a multiplex assay system based on real-time reverse transcription polymerase chain reaction (rRT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in upper respiratory specimens (such as nasal, mid-turbinate, nasopharyngeal, oropharyngeal swab specimens and nasopharyngeal wash/aspirate or nasal aspirate specimens) collected from individuals suspected of COVID-19 by their healthcare provider. The AMPIPROBE SARS-CoV-2 Test System has been authorized by FDA under an Emergency Use Authorization (EUA). It is adaptable to different extraction methodologies and throughput including our proprietary GENFLEX® Platform, QIAsymphony® SP, and manual workflow.
Performance:
- Sensitivity*: 96.2%
- Specificity*: 98.0%
- Time to results: 192 samples in one shift
*Percent agreements between the AMPIPROBE® Test System and an FDA-approved EUA test performed on nasopharyngeal swabs
 Figure 1.
Figure 1. AMPIPROBE® SARS-CoV-2 Test System on the GENFLEX® platform
Our AMPIPROBE® SARS-CoV-2 Test System is designed to also be used in modular settings according to customer needs and consistent with the FDA EUA-approved directions for use. Each of the workflow components can be combined with any equipment specifically listed in the directions for use.
AMPIXTRACT™ SARS-CoV-2 Extraction Kit
 |
|
Performance
- Magnetic bead separation technology with proprietary buffer system
- Isolation of high-quality RNA in ≺ 1 hour
|
Download our Flyer:
Extraction Kit for SARS-CoV-2 - EUA
AMPIPROBE® SARS-CoV-2 Assay Kit
Download our Flyer:
Detection Kit for SARS-CoV-2 - EUA
AMPIPROBE® SARS-CoV-2 Controls
The AMPIPROBE® SARS-CoV-2 Controls is part of our AMPIPROBE® SARS-CoV-2 Test System and is intended to be used in combination with AMPIPROBE® SARS-CoV-2 Assay kit and AMPIXTRACT™ SARS-CoV-2 Extraction Kit during the PCR process. The AMPIPROBE® SARS-CoV-2 Controls Kit contains a positive RNA control that is specific to the SARS-CoV-2 genomic regions targeted by the assay as well as human RNase P, a negative RNA control that contains target to human RNase P, and a no-template control (NTC).
Summary of SARS-CoV-2 Reagents and Instruments Combination for COVID-19 Molecular Testing
Emergency Authorization Use Only
In Vitro Diagnostic (IVD) Use Only
Prescription/Rx Use Only
The AMPIPROBE® SARS-CoV-2 Test System has not been FDA cleared or approved; the test has been authorized by FDA under an Emergency Use Authorization (EUA) for use by laboratories certified under the Clinical Laboratory Improvement Amendments (CLIA) of 1988, 42 U.S.C. §263a, to perform high complexity tests.
The AMPIPROBE® SARS-CoV-2 Test System has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens.
The AMPIPROBE® SARS-CoV-2 Test System is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
All product names, logos, and brands are the property of their respective owners.
AMPIPROBE® SARS-CoV-2 Test System (RUO)
Our SARS-CoV-2 Test System is designed to also be used in open modular settings according to customer needs. Each of the workflow components can be combined with any open platforms or used in manual settings.
GENFLEX® platform
|
OR |
- Any open liquid handling platform
- Any qPCR instrument
|
AMPIXTRACT™ SARS-CoV-2 Extraction Kit (RUO)
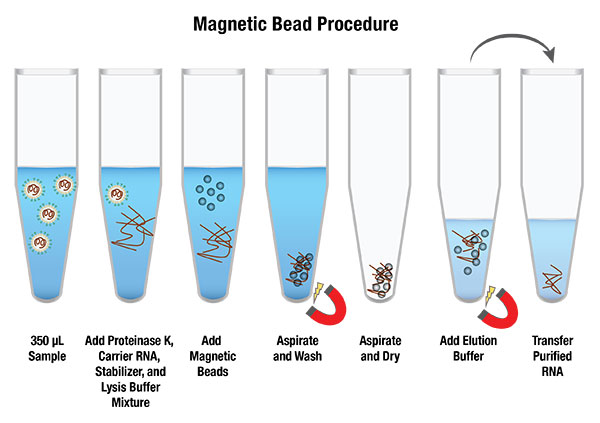
Our AMPIXTRACT™ SARS-CoV-2 Extraction Kit (RUO) is designed for isolating viral RNA from nasopharyngeal samples. The isolated RNA is pure and ready for your downstream application including direct input to the target amplification process through RT-PCR methods. The flexible kit design is easily adapted to both automated, high-throughput and manual processing.
Performance
- Magnetic bead separation technology with proprietary buffer system
- Isolation of high-quality RNA in < 1 hour
- > 99% recovery of extracted RNA from 350 μL sample
Easily Adaptable
- Manual or automated processing
- Compatible with common automated open platforms and RT-PCR kits
- Flexible for 1.5 mL tubes or 96-well plates
Download our Flyer:
Extraction Kit for SARS-CoV-2 - RUO
AMPIPROBE® SARS-CoV-2 Assay Kit (RUO)
Our AMPIPROBE® SARS-CoV-2 Assay Kit (RUO) is a multiplexed assay designed for the detection of the SARS-CoV-2 virus. It contains two primer/probe sets specific to different SARS-CoV-2 genomic regions and primers/probes for internal control and negative control, human RNase P. This kit is intended to be used in combination with general RNA extraction kits, including AMPIXTRACT™ SARS-CoV-2 Extraction Kit (RUO) and generic qPCR instruments. This assay can also be used in manual settings or with open automated platforms such as the GENFLEX® Automated Instrument (please contact us for more information) or any other open platforms available on the market for the liquid handling process of RNA extraction and PCR set up.
Performance
- Sensitivity: 98.1%
- Clinical Specificity: 99.3%
- Size: 96 tests
Easily Adaptable
- Adaptable to both automated open platforms (including GENFLEX®) and manual processing
Cost-Effective
- Low-cost alternative to other methods of viral load detection
AMPIPROBE® SARS-CoV-2 Assay Kit (RUO) was validated on the following RT-qPCR instruments:
- QuantStudio® 5 (Applied Biosystems®)
- Rotogene® (QIAGEN®)
- qTOWER (Analytik Jena®)
Note: Extraction of RNA was performed either with the GENFLEX® Platform using AMPIXTRACT™ SARS-CoV-2 Extraction Kit (RUO) or with QIAsymphony SP (Qiagen®) using QIAsymphony DSP Virus/Pathogen Midi Kit(Qiagen®)
Download our Flyer:
Detection Kit for SARS-CoV-2 - RUO
AMPIPROBE® SARS-CoV-2 Controls (RUO)
Build Your Own COVID-19 Molecular Detection Assay
Another alternative solution for our customers is the ability to build their own molecular detection kit by combining our reagents for RT-PCR with primers of their choice.
Our AMPIGENE® 1-Step RT-PCR Kit uses the latest developments in reverse transcriptase (RT) technology and buffer chemistry for efficient cDNA synthesis and PCR in a single tube. An optimized buffer system allows efficient amplification, and proprietary enhancers prevent the formation of primer dimers, to increase sensitivity and specificity.
- Convenient one tube reaction (cDNA synthesis and qPCR in a single tube)
- Thermostable and extremely active RTase
- For dye-based and probe-based qPCR applications
- Flexible: can be adapted to a variety of platforms
- 200 and 1000 reactions formats available
We offer this product as a dye-based kit or as a probe-based kit. In both cases, our customers are only required to provide specific SARS-CoV-2 primers or SARS-CoV-2 labelled probes, respectively, to build a complete COVID-19 detection kit.
Complementary PCR Products