- A simple, sensitive, homogenous fluorescence assay with no need for sample separation or dilution
- Able to optimize protein stability conditions in an accelerated manner
- Can detect ligand binding, even without a prior knowledge of the protein’s function or ligand binding site
- Works over a wide temperature, pH and ionic strength range and compatible with commonly used buffers and excipients
- Provides a convenient, complementary orthogonal method for cross-validation of instrumentation-intensive techniques
The PROTEOSTAT® Thermal shift stability assay kit includes a fluorescent dye which detects protein aggregation, so it can be used to monitor protein stability under systematic thermal stress conditions. From the thermal shift assay, a temperature at which the bulk of the protein becomes aggregated can readily be identified. The aggregation temperature is an indicator of protein stability and can be used to optimize conditions that minimize protein aggregation as well as to identify ligands that bind and confer structural stability to a protein of interest. Use the related PROTEOSTAT® Protein Refolding and Aggregation Sensing Kit (Prod. No. ENZ-51040) to identify optimal protein refolding conditions. Conditions that increase the aggregation temperature, increase the stability of the protein.
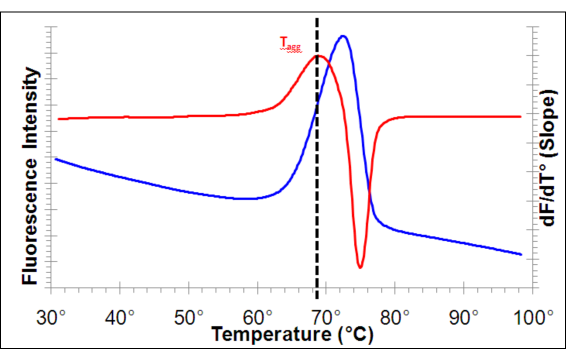
Figure 1. Typical results of the PROTEOSTAT® Thermal Shift Stability Assay are shown for goat anti-mouse IgG (11.2 mg/ml at pH 7.4). Using a RT-PCR instrument programmed to ramp the temperature from 30° to 99°C at a 3°/ minute rate, while reading the fluorescence continuously. The blue line represents the raw fluorescence data, and the red line shows the corresponding first derivative trace, highlighting the slope of the fluorescence intensity curve. The first derivative plot provides the aggregation temperature of the protein (Tagg: The point of maximal slope).

Figure 2. PROTEOSTAT® Dye’s Aggregation Temperature is not equivalent to the Melting Temperature Obtained Using SYPRO® Orange.
SYPRO Orange dye is an environment-sensitive dye that binds to the hydrophobic regions of unfolded proteins. It can be used to measure Tm. Tm is the temperature mid-point of a thermal unfolding curve. At the temperature midpoint Tm, one half of the target proteins in a sample are unfolded, and one half of the target proteins in the sample remain folded. Unfolding does not depend upon neighboring proteins. ProteoStat is a molecular rotor dye that binds to the surface of protein aggregates. Tagg refers to the mid-point of a thermal aggregation curve. Proteins unfold and then subsequently aggregate. Aggregation depends upon neighboring proteins. The higher the protein concentration, the greater the tendency to aggregate.
Please mouse over
Product Details
| Application Notes: | This kit has been designed for monitoring protein stability under systematic thermal stress conditions. |
| |
| Quality Control: | A sample kit from each lot of PROTEOSTAT® Thermal shift stability assay kit is tested using the procedure described in the manual. The detected aggregation temperature for 16µg/ml of β-lactoglobulin is a single peak at 76° ± 2°C. |
| |
| Use/Stability: | With proper storage, the kit components are stable up to the date noted on the product label. Store kit at -20˚C in a non-frost free freezer, or –80˚C for longer term storage. |
| |
| Handling: | Protect from light. Avoid freeze/thaw cycles. |
| |
| Shipping: | Blue Ice |
| |
| Short Term Storage: | -20°C |
| |
| Long Term Storage: | -20°C |
| |
| Contents: | PROTEOSTAT® TS Detection Reagent
β-Lactoglobulin control
10X Assay Buffer |
| |
| Technical Info/Product Notes: | Application Note:
Prediction of Aggregation Propensity and Monitoring of Aggregation of Antibody-Drug Conjugates (ADC) using ProteoStat® Reagents
Cited samples:
PROTEOSTAT® Cited Samples
Enzo and PROTEOSTAT are trademarks of Enzo Life Sciences, Inc. Grenier is a registered trademark of Grenier Bio-One. Several of Enzo’s products and product applications are covered by US and foreign patents and patents pending. This product is manufactured and sold by ENZO LIFE SCIENCES, INC. for research use only by the end-user in the research market and is not intended for diagnostic or therapeutic use. Purchase does not include any right or license to use, develop or otherwise exploit this product commercially. Any commercial use, development or exploitation of this product or development using this product without the express prior written authorization of ENZO LIFE SCIENCES, INC. is strictly prohibited. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
Constructive approach for synthesis of a functional IgG using a reconstituted cell-free protein synthesis system: S. Murakami, et al.; Sci. Rep.
9, 671 (2019),
Application(s): Purified IgG or Trastuzumab,
Abstract;
Full Text
Structural insights into humanization of anti-tissue factor antibody 10H10: A. Teplyakov, et al.; MAbs.
10, 269 (2018),
Application(s): Monoclonal antibodies,
Abstract;
Full Text
Protein catalyzed capture agents with tailored performance for in vitro and in vivo applications: M.B. Coppock, et al.; Biopolymers
108, e22934 (2017),
Application(s): Stability of peptide-based ligands,
Abstract;
Time-Dependent Protein Thermostability Assay: I. Vandecaetsbeek, et al.; Methods Mol. Biol.
1377, 79 (2016),
Abstract;
Effect of Polyethylene Glycol Conjugation on Conformational and Colloidal Stability of a Monoclonal Antibody Antigen-Binding Fragment (Fab'): C. Roque, et al.; Mol. Pharm.
12, 562 (2015),
Abstract;
Modulating the thermostability of Endoglucanase I from Trichoderma reesei using computational approaches: G. Bayram Akcapinar, et al.; Protein Eng. Des. Sel.
28, 127 (2015),
Abstract;
Site-specific conjugation of monomethyl auristatin E to anti-CD30 antibodies improves their pharmacokinetics and therapeutic index in rodent models: F. Lhospice, et al.; Mol. Pharm.
12, 1863 (2015),
Abstract;
A general approach to antibody thermostabilization: A.D. McConnell, et al.; MAbs
6, 1274 (2014),
Application(s): Stability of antibodies,
Abstract;
Full Text
Modifications of cysteine residues in the transmembrane and cytoplasmic domains of a recombinant hemagglutinin protein prevents cross-linked multimer formation and potency loss: K.M. Holtz, et al.; BMC Biotechnol.
14, 111 (2014),
Abstract;
An integrated approach to extreme thermostabilization and affinity maturation of an antibody: A.D. McConnell, et al.; Protein Eng. Des. Sel.
26, 151 (2013),
Abstract;
Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid: J. Conlon, et al.; J. Immunol.
190, 5216 (2013),
Abstract;
Optimization of protein purification and characterization using Thermofluor screens: S. Boivin, et al.; Protein Expr. Purif.
91, 192 (2013), (Review),
Abstract;
Protein quality control acts on folding intermediates to shape the effects of mutations on organismal fitness: S. Bershtein, et al.; Mol. Cell
49, 133 (2013),
Application(s): Aggregation propensity of the DHFR proteins,
Abstract;
General Literature References
Estimation of protein aggregation propensity with a melting point apparatus: A.A. Raibekas; Anal. Biochem.
380, 331 (2008),
Abstract;
Thermofluor-based high-throughput stability optimization of proteins for structural studies: U.B. Ericsson, et al.; Anal. Biochem.
357, 289 (2006),
Abstract;
Structure of the cross-beta spine of amyloid-like fibrils: R. Nelson, et al.; Nature
435, 773 (2005),
Abstract;
Study of the thermal denaturation of ribonuclease A by differential thermal analysis and susceptibility to proteolysis: B.G. Winchester, et al.; Biochem J.
117, 299 (1970),
Abstract;
Related Products

















