- 10X more sensitive than JC-1 with superior aqueous solubility
- Photostable dual-emission dye
- Suitable for time-course studies evaluating intact and compromised mitochondria
- No-wash/No-medium removal
- Separate MITO-ID® assay is available for detection of mitochondrial mass
- Detects toxicity at lower drug/dose concentrations
- No solvent artifacts as those seen with JC-1 formulation
- Suitable for high-throughput applications
The MITO-ID® Membrane Potential Cytotoxicity Kit measures fluctuations in mitochondrial membrane potential (MMP) utilizing a cationic dual-emission dye that exists as green fluorescent monomers in the cytosol, and accumulates as orange fluorescent J-aggregates in the mitochondria. Mitochondria having a low membrane potential will accumulate low concentrations of dye and will exhibit green fluorescence while more highly polarized mitochondria will exhibit orange-red fluorescence. Cells exhibit a shift from orange to green fluorescence as mitochondrial function becomes increasingly compromised. The kit is a unique HTS assay that monitors mitochondrial membrane potential in real-time without the need for washes or medium removal.
Mechanism of Action
The basic chemical structure of the dye consists of highly conjugated moieties that extensively delocalize a positive charge thus allowing electrophoretic uptake toward the negatively charged matrix phase of the polarized inner mitochondrial membrane. The dye is capable of entering selectively into mitochondria wherein it changes its color reversibly from green to orange as membrane potential increases (dual-emission potential probe). This photophysical property is due to the reversible formation of J-aggregates upon membrane polarization that causes shifts in emitted light from ~530nm (the emission of the monomeric dye) to 590 nm (the emission of the J-aggregate form) when excited at 490 nm. As a consequence, mitochondria having a low membrane potential will accumulate low concentrations of dye and will exhibit green fluorescence while more highly polarized mitochondria will exhibit orange fluorescence.
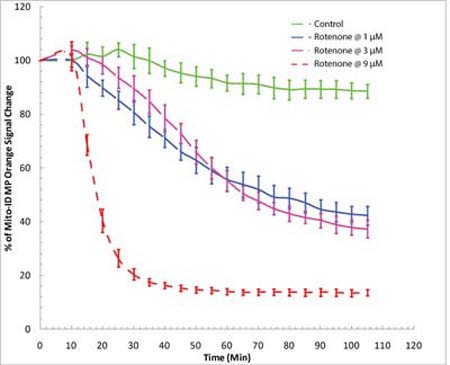
Real-time detection of mitotoxicity in drug screening. Time-course study of mitochondrial membrane potential changes using a BioTek Synergy™ Mx fluorescence microplate reader. HeLa cells were incubated with MITO-ID® MP dye for 30 minutes at room temperature (no serum or media removal). Rotenone was added to achieve concentrations of 1 µM, 3 µM and 9 µM. MITO-ID® MP dye was shown to be responsive to rotenone, as demonstrated by a decrease in orange signal.
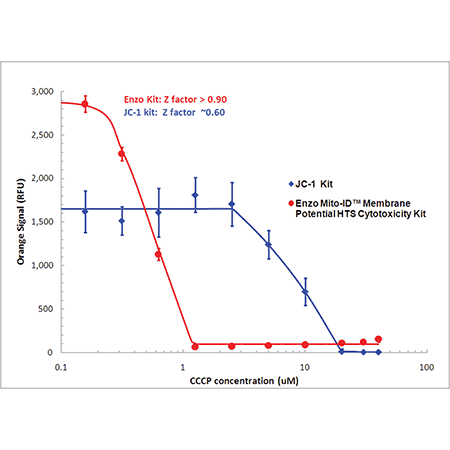
Detect mitochondrial perturbations with 10 times more sensitivity than JC-1. Mitochondrial membrane potential (MMP) was evaluated in HeLa cells treated with CCCP using MITO-ID® dye (red) or JC-1 (blue). Using a conventional fluorescence microplate reader, MMP was shown to decrease with increasing CCCP concentration as indicated by a decrease in orange fluorescence. Improved aqueous solubility of the dye and no-wash protocol minimizes variability, leading to a higher Z-factor (> 0.9) than that obtained with JC-1.
Please mouse over
Product Details
| Applications: | HTS
Microplate
|
| |
| Application Notes: | MITO-ID® Membrane Potential Cytotoxicity Kit enables monitoring of mitochondrial potential changes using a simple fluorescence microplate reader. |
| |
| Quality Control: | A sample kit from each lot of MITO-ID® Membrane Potential Cytotoxicity Kit is assayed using the microplate-based assay described in the manual. The Z’-factor from CCCP-treated cells is greater than 0.6. |
| |
| Quantity: | For 2 x 96-well microplates |
| |
| Handling: | Protect from light. Avoid freeze/thaw cycles. |
| |
| Shipping: | Dry Ice |
| |
| Short Term Storage: | -20°C |
| |
| Long Term Storage: | -80°C |
| |
| Contents: | MITO-ID® MP Detection Reagent, 200 μL
CCCP Control, 100 μL
10X Assay Buffer 1: 2.5 mL
50X Assay Buffer 2: 0.5 mL |
| |
| Scientific Background: | Mitochondria play a central role in cellular metabolism, bioenergetics, and apoptosis. Decreased mitochondrial function is known to be a major contributor to drug-associated toxicity in various organs. Growing FDA emphasis on evaluating the mitotoxic effects of drug candidates has increased the importance of determining such effects early in the drug development process. |
| |
| Technical Info/Product Notes: | The MITO-ID® Membrane Potential Cytotoxicity Kit is a member of the CELLESTIAL® product line, reagents and assay kits comprising fluorescent molecular probes that have been extensively benchmarked for live cell analysis applications. CELLESTIAL® reagents and kits are optimal for use in demanding imaging applications, such as confocal microscopy, flow cytometry and HCS, where consistency and reproducibility are required. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management: M. Pellei, et al.; Int. J. Mol. Sci.
24, 4091 (2023),
Abstract;
FUNDC1 regulates receptor-mediated mitophagy independently of the PINK1/Parkin-dependent pathway in rotenone-treated SH-SY5Y cells: S.Y. Park, et al.; Food Chem. Toxicol.
137, 111163 (2020),
Application(s): Fluorescence microscopy and microplate reader,
Abstract;
Inhibitory role of TRIP-Br1/XIAP in necroptosis under nutrient/serum starvation: Z. Sandag, et al.; Mol. Cells
43, 236 (2020),
Abstract;
Full Text
NAD hydrolysis by the tuberculosis necrotizing toxin induces lethal oxidative stress in macrophages: D. Pajuelo, et al.; Cell. Microbiol.
22, e13115 (2020),
Application(s): THP-1 macrophages; microplate reader,
Abstract;
Full Text
Impaired autophagic and mitochondrial functions are partially restored by ERT in Gaucher and Fabry diseases: M.M. Ivanova, et al.; PLoS One
14, e0210617 (2019),
Application(s): Fluorescence microscopy,
Abstract;
Full Text
Inhibitory role of AMP‑activated protein kinase in necroptosis of HCT116 colon cancer cells with p53 null mutation under nutrient starvation: D.T. Le, et al.; Int. J. Oncol.
54, 702 (2019),
Abstract;
ROS as a novel indicator to predict anticancer drug efficacy: T. Zaidieh, et al.; BMC Cancer
19, 1224 (2019),
Abstract;
Full Text
TREM1/3 deficiency impairs tissue repair after acute kidney injury and mitochondrial metabolic flexibility in tubular epithelial cells: A. Tammaro, et al.; Front. Immunol.
10, 1469 (2019),
Application(s): Microplate reader,
Abstract;
Full Text
Anti-cancerous effect of cis-khellactone from Angelica amurensis through the induction of three programmed cell deaths: S. Jung, et al.; Oncotarget
9, 16744 (2018),
Application(s): Microplate reader,
Abstract;
Full Text
Blue light phototoxicity toward human corneal and conjunctival epithelial cells in basal and hyperosmolar conditions: V. Marek, et al.; Free Radic. Biol. Med.
126, 27 (2018),
Application(s): Microplate reader,
Abstract;
cGAS drives noncanonical-inflammasome activation in age-related macular degeneration: N. Kerur, et al.; Nat. Med.
24, 50 (2018),
Abstract;
Full Text
Cytotoxicity of propofol in human induced pluripotent stem cell-derived cardiomyocytes: K. Kido, et al.; J. Anesth.
32, 120 (2018),
Application(s): Microplate reader,
Abstract;
Full Text
Development of novel amino-quinoline-5, 8-dione derivatives as NAD (P) H: quinone oxidoreductase 1 (NQO1) inhibitors with potent antiproliferative activities: Y. Ling, et al.; Eur. J. Med. Chem.
154, 199 (2018),
Abstract;
Full Text
Inhibition of apoptosis using exosomes in Chinese hamster ovary cell culture: S. Han, et al.; Biotechnol. Bioeng.
115, 1331 (2018),
Abstract;
Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells: M. Marie, et al.; Cell Death Disc.
9, 287 (2018),
Abstract;
Full Text
Clearance of damaged mitochondria through PINK1 stabilization by JNK and ERK MAPK signaling in chlorpyrifos-treated neuroblastoma cells: J.H. Park, et al.; Mol. Neurobiol.
54, 1844 (2017),
Abstract;
PGAM5 regulates PINK1/Parkin-mediated mitophagy via DRP1 in CCCP-induced mitochondrial dysfunction: Y.S. Park, et al.; Toxicol. Lett.
284, 120 (2017),
Abstract;
Absence of selenium protection against methylmercury toxicity in harbour seal leucocytes in vitro: K. Das, et al.; Mar. Pollut. Bull.
108, 70 (2016),
Application(s): Measured the mitochondrial membrane potential,
Abstract;
Decreased poly(ADP-ribose) polymerase 1 expression attenuates glucose oxidase-induced damage in rat cochlear marginal strial cells: Y. Zhang, et al.; Mol. Neurobiol.
53, 5971 (2016),
Abstract;
PPAR-γ activation attenuates deltamethrin-induced apoptosis by regulating cytosolic PINK1 and inhibiting mitochondrial dysfunction: K. Juyeon, et al.; Toxicol. Lett.
260, 8 (2016),
Application(s): Changes in mitochondrial membrane potential (MMP), SH-SY5Y cells,
Abstract;
Cytotoxicity assessment of graphene-based nanomaterials on human dental follicle stem cells: D. Olteanu, et al.; Colloids Surf. B Biointerfaces
136, 791 (2015),
Abstract;
Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell-derived neurons: H. Ito, et al.; PLoS One
10, e0128445 (2015),
Application(s): Mitochondria membrane potential in human iPSC-derived neurons,
Abstract;
Full Text
A mitochondrial thioredoxin-sensitive mechanism regulates TGF-β-mediated gene expression associated with epithelial-mesenchymal transition: F. Ishikawa, et al.; Biochem. Biophys. Res. Commun.
443, 821 (2014),
Abstract;
Stressed cybrids model demyelinated axons in multiple sclerosis: L. Llobel, et al.; Metab. Brain Dis.
28, 639 (2013),
Abstract;
TLR4 activation under lipotoxic conditions leads to synergistic macrophage cell death through a TRIF-dependent pathway: J.D. Schilling, et al.; J. Immunol.
190, 1285 (2013),
Abstract;
Full Text
Coenzyme depletion by members of the aerolysin family of pore-forming toxins leads to diminished ATP levels and cell death: C.M. Fennessey, et al.; Mol. Biosys.
8, 2097 (2012),
Abstract;
Dengue virus utilizes calcium modulating cyclophilin-binding ligand to subvert apoptosis: J. Li, et al.; BBRC
418, 622 (2012),
Application(s): Detection of membrane potential in Dengue virus-infected cells,
Abstract;
Hormone-induced 14-3-3γ adaptor protein regulates steroidogenic acute regulatory protein activity and steroid biosynthesis in MA-10 Leydig cells: Y. Aghazadeh, et al.; J. Biol. Chem.
287, 15380 (2012),
Application(s): Mitochondrial staining of MA-10 mouse Leydig tumor cells,
Abstract;
Full Text
General Literature References
High throughput fluorescence assays for the measurement of mitochondrial activity in intact human neuroblastoma cells: A.J. Woollacott & P.B. Simpson; J. Biomol. Screen
6, 413 (2001),
Abstract;
A New Method for the Cytofluorometric Analysis of Mitochondrial Membrane Potential Using the J-Aggregate Forming Lipophilic Cation 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine Iodide (JC-1): A. Cossarizza, et al.; BBRC
197, 40 (1993),
Abstract;
Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate forming lipophilic cation JC-1: S.T. Smiley, et al.; PNAS
88, 3671 (1991),
Full Text
Related Products