-
Qualitative assessment of an Ub E3 ligase enzyme’s activity through its ability to auto-ubiquitinylate.
-
Testing of proteins for auto-ubiquitinylation activity allowing their identification as putative ubiquitin E3 ligases.
-
Ubiquitinylation of substrate proteins (user provided) specific to a particular ubiquitin E3 ligase.
This E3 ligase auto-ubiquitinylation kit enables proteins to be tested for ubiquitin E3 ligase activity through assessment of their ability to under auto-ubiquitinylation. Utilizing the first three steps in the ubiquitin cascade the kit facilitates ubiquitinylation of known or putative E3 ligase enzymes followed by Western blot analysis using the highly sensitive reagents provided or using antibodies to the specific protein of interest (user supplied). A high integrity ubiquitin E3 ligase enzyme is also provided for use as a positive control.
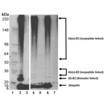
Western blot analysis of control Ub E3 ligase Hdm2 RING domain auto-ubiquitinylation assays. Auto-ubiquitinylation assays set-up and run as described in “Assay protocol”. Ubiquitinylated E3 ligase species were detected by Western blotting as described in “Analysis by western blotting”, using the provided ubiquitin antibody (Prod. No. ADI-SPA-203) at a dilution of 1:1,000. Results demonstrate auto-ubiquitinylation of the control Hdm2 RING domain ligase under the given assay conditions. (E2-Ub (thioester linked) is removed in reducing condition. Left – non-reducing; Right – reducing condition.)
Lane 1: Pre-stained Protein Standard. Lane 2: Hdm2-Ub, (+) control, (non-reduced). Lane 3: Hdm2, (-) control, (no Mg-ATP), (non-reduced). Lane 4: Hdm2-Ub, (+) control, (reduced). Lane 5: Hdm2, (-) control, (no Mg-ATP), (reduced). Lane 6: Hdm2-Ub, (+) control, (reduced). Lane 7: Hdm2, (-) control (no ubiquitin), (reduced).
Please mouse over
Product Details
| Application Notes: | For assessment of protein Ub E3 ligase activity. |
| |
| Quantity: | Sufficient for 10 auto-ubiquitinylation assays. |
| |
| Handling: | Avoid freeze/thaw cycles. |
| |
| Shipping: | Dry Ice |
| |
| Short Term Storage: | -80°C |
| |
| Long Term Storage: | -80°C |
| |
| Contents: | 20X Ubiquitin Activating Enzyme Solution (E1), 25µl
20X Ubiquitin Conjugating Enzyme Solution (E2), 25µl
10X Ubiquitin Solution (Ub), 50µl
20X Control Ubiquitin Ligase Solution (E3), 25µl
20X Mg-ATP Solution, 25µl
10X Ub E3 ligase Buffer, 50µl
Ubiquitin antibody solution, 25µl |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
Expression quantitative trait loci mapping identified PtrXB38 as a key hub gene in adventitious root development in Populus: T. Yao, et al.; New Phytol. (2023),
Abstract;
Overexpression of ARM repeat/U-box containing E3 ligase, PUB2 positively regulates growth and oxidative stress response in Arabidopsis: L.K. Saini, et al.; Biochem. J.
480, 555 (2023),
Abstract;
p21-activated kinase 4 controls the aggregation of α-synuclein by reducing the monomeric and aggregated forms of α-synuclein: involvement of the E3 ubiquitin ligase NEDD4-1: S.Y. Won, et al.; Cell Death Dis.
13, 575 (2022),
Abstract;
Arabidopsis thaliana Ubiquitin-Associated Protein 1 (AtUAP1) Interacts with Arabidopsis thaliana Ring Zinc Finger 1 (AtRZF1) to Negatively Regulate Dehydration Response: J.H. Min, et al.; Plant Cell Physiol.
62, 1044 (2021),
Abstract;
PLPP/CIN-mediated Mdm2 dephosphorylation increases seizure susceptibility via abrogating PSD95 ubiquitination: J.E. Kim, et al. ; Exp. Neurol.
331, 113383 (2020),
Abstract;
Genome-wide identification of genes encoding putative secreted E3 ubiquitin ligases and functional characterization of PbRING1 in the biotrophic protist Plasmodiophora brassicae: F. Yu, et al.; Curr. Genet.
65, 1355 (2019),
Abstract;
PAMP-responsive ATL gene StRFP1 and its orthologue NbATL60 positively regulate Phytophthora infestans resistance in potato and Nicotiana benthamiana: C. Zhong, et al.; Plant Sci.
270, 47 (2018),
Abstract;
Ubiquitin-dependent proteolysis of CXCL7 leads to posterior longitudinal ligament ossification: M. Tsuru, et al.; PLoS One
13, e0196204 (2018),
Abstract;
Full Text
The F-box Protein KIB1 Mediates Brassinosteroid-Induced Inactivation and Degradation of GSK3-like Kinases in Arabidopsis: J.Y. Zhu, et al.; Mol. Cell
66, 648 (2017),
Abstract;
Full Text
Cloning and characterization of carboxyl terminus of heat shock cognate 70-interacting protein gene from the silkworm, Bombyx mori: T. Oshawa, et al.; Comp. Biochem. Physiol. B. Biochem. Mol. Biol.
201, 29 (2016),
Application(s): In vitro ubiquitination assay, human proteins,
Abstract;
U-box E3 ubiquitin ligase PUB17 acts in the nucleus to promote specific immune pathways triggered by Phytophthora infestans: Q. He, et al.; J. Exp. Bot.
66, 3189 (2015),
Application(s): Assay,
Abstract;
Full Text
FOXL2 posttranslational modifications mediated by GSK3β determine the growth of granulosa cell tumours: J.H. Fox, et al.; Nat. Commun.
5, 2936 (2014),
Abstract;
Heterologous expression of the gourd E3 ubiquitin ligase gene LsRZF1 compromises the drought stress tolerance in Arabidopsis thaliana: J.H. Min, et al.; Plant Physiol. Biochem.
77, 7 (2014),
Abstract;
Trim32 facilitates degradation of MYCN on spindle poles and induces asymmetric cell division in human neuroblastoma cells: H. Izumi, et al.; Cancer Res.
74, 5620 (2014),
Abstract;
Effect of ursolic acid on MAPK in cyclin D1 signaling and RING-type E3 ligase (SCF E3s) in two endometrial cancer cell lines: Y. Achiwa, et al.; Nutr. Cancer
65, 1026 (2013),
Abstract;
The atrzf1 mutation of the novel RING-type E3 ubiquitin ligase increases proline contents and enhances drought tolerance in Arabidopsis: H.W. Ju, et al.; Plant Sci.
203, 1 (2013),
Abstract;
General Literature References
The UBXN1 protein associates with Autoubiquitinated forms of the BRCA1 tumor suppressor and inhibits its enzymatic function: F. Wu-Baer, et al.; Mol. Cell Biol.
30, 2787 (2010),
Abstract;
Autoubiquitination of BCA2 RING E3 ligase regulates its own stability and affects cell migration: Y. Amemiya, et al.; Mol. Cancer Res.
6, 1385 (2008),
Abstract;
Mechanisms underlying ubiquitination: C.M. Pickart; Annu. Rev. Biochem.
70, 503 (2001),
Abstract;
The ubiquitin-proteasome system and endocytosis: G.J. Strous & R. Govers; J. Cell Sci.
112 (Pt 10), 1417 (1999),
Abstract;
The ubiquitin system: A. Hershko & A. Ciechanover; Annu. Rev. Biochem.
67, 425 (1998),
Abstract;
Pathways of ubiquitin conjugation: A.L. Haas & T.J. Siepmann; FASEB J.
11, 1257 (1997),
Abstract;