Product Details
| Alternative Name: | Histone deacetylase 6 |
| |
| MW: | 131 kDa |
| |
| Source: | Produced in insect cells. Produced in a baculovirus expression system. |
| |
| UniProt ID: | Q9UBN7 |
| |
| Gene/Protein Identifier: | NM_006044 (RefSeq) |
| |
| Formulation: | Liquid. In 50mM TRIS, pH 8.0, 138 mM NaCl and 10% glycerol. |
| |
| Purity Detail: | Partially purified by single-step affinity chromatography and gel filtration. |
| |
| Biological Activity: | Active with various fluorogenic peptide substrates, including FLUOR DE LYS® deacetylase substrate (Prod. No. BML-KI104) and FLUOR DE LYS®-SIRT1 deacetylase substrate (Prod. No. BML-KI177). |
| |
| Specific Activity: | ≥1 U/µg. One U=1 pmol/min at 37°C, 100 µM, FLUOR DE LYS®-SIRT1 deacetylase substrate (Prod. No. BML-KI177). |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Scientific Background: | HDAC6 is a class II HDAC, a group defined by its member’s homology to the yeast HDAC HDA1. It is unique among human HDACs in that it contains two full deacetylase domains. A tubulin deacetylase, HDAC6 may act to promote autophagic clearing of huntingtin aggregates and retard HIV1 infection, but also to promote invasive motility in tumor cells. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
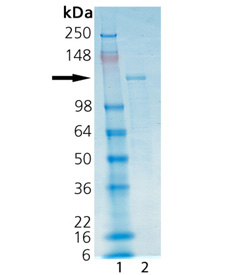
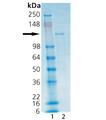
SDS-PAGE analysis: Lane 1: MW marker, Lane 2: 2µg HDAC6.
Please mouse over
Product Literature References
Novel hydroxamic acid derivative induces apoptosis and constrains autophagy in leukemic cells: M.A. Fischer, et al.; J. Adv. Res. (2023),
Abstract;
Design, Synthesis, and biological evaluation of HDAC6 inhibitors based on Cap modification strategy: X. Li, et al.; Bioorg. Chem.
125, 105874 (2022),
Abstract;
Discovery of Novel Src Homology-2 Domain-Containing Phosphatase 2 and Histone Deacetylase Dual Inhibitors with Potent Antitumor Efficacy and Enhanced Antitumor Immunity: M. Liu, et al.; J. Med. Chem.
65, 12200 (2022),
Abstract;
New 5-Aryl-Substituted 2-Aminobenzamide-Type HDAC Inhibitors with a Diketopiperazine Group and Their Ameliorating Effects on Ischemia-Induced Neuronal Cell Death: Y. Hirata, et al.; Sci. Rep.
8, 1400 (2018),
Abstract;
4-(1-Ethyl-4-anisyl-imidazol-5-yl)-N-hydroxycinnamide–A new pleiotropic HDAC inhibitor targeting Cancer cell signalling and cytoskeletal organisation: K. Mahal, et al.; Exp. Cell Res.
336, 263 (2015),
Application(s): HDAC Assay,
Abstract;
Tasquinimod is an allosteric modulator of HDAC4 survival signaling within the compromised cancer microenvironment: J.T. Isaacs, et al.; Cancer Res.
73, 1386 (2012),
Abstract;
Tubulin acetylation alone does not affect kinesin-1 velocity and run length in vitro: W.J. Walter, et al.; PLoS One
7, e42218 (2012),
Abstract;
Full Text
Cutting edge: tubulin a functions as an adaptor in NFAT-importin b interaction: K. Ishiguro, et al.; J. Immunol.
186, 2710 (2011),
Abstract;
Full Text
Synthesis and evaluation of aliphatic-chain hydroxamates capped with osthole derivatives as histone deacetylase inhibitors: W.H. Huang, et al.; Eur. J. Med. Chem.
46, 4042 (2011),
Abstract;
Related Products