- Naturally-occurring active form of MMP-9
- High purity
- High activity
Product Details
| Alternative Name: | Matrix metalloproteinase 9, Gelatinase B, 92 kDa Type IV collagenase |
| |
| MW: | 39 kDa |
| |
| Source: | Produced in E. coli. Active recombinant matrix metalloproteinase-9 (MMP-9, gelatinase B, 92 kDa type IV collagenase) cloned from human cDNA. The enzyme consists of residues Phe107-Pro449 (NM_004994), which comprises the catalytic/fibronectin domain of human MMP-9, with a C-terminal purification tag. This represents a naturally-occurring active form of MMP-9 which lacks the C-terminal hemopexin domain. Activity toward its targets, such as gelatin, casein, or peptide substrates, is unaffected. |
| |
| UniProt ID: | P14780 |
| |
| Formulation: | Liquid. In 50mM TRIS, pH 7.5, containing 1mM calcium chloride, 300mM sodium chloride, 5μM zinc chloride, 0.1% Brij-35 and 15% glycerol. |
| |
| Purity: | ≥95% (SDS-PAGE) |
| |
| Purity Detail: | Purified by multi-step chromatography. |
| |
| Activity: | Yes |
| |
| Specific Activity: | ≥500 pmol/min/ug at 37°C using the colorimetric thiopeptolide Ac-Pro-Leu-Gly-S-Leu-Leu-Gly-OEt (100 µM; Prod. No. BML-P125) as substrate. |
| |
| Application Notes: | Useful tool to study enzyme kinetics, cleave target substrates, and screen for inhibitors. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Handling: | Avoid freeze/thaw cycles. After opening, prepare aliquots and store at -80°C. |
| |
| Scientific Background: | Matrix metallopeptidase 9 (MMP-9) belongs to a class of enzymes that belong to the zinc-metalloproteinase family involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, angiogenesis, bone development, wound healing, cell migration, learning and memory. Its also associated with numerous pathological processes, including cancer, immunologic and cardiovascular diseases. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
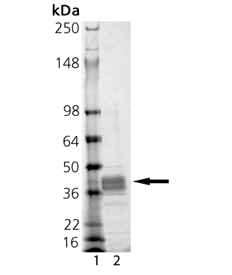
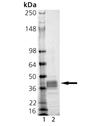
SDS-PAGE Analysis: Lane 1: MW Marker, Lane 2: 1μg MMP-9 (catalytic domain) (human), (recombinant, E. coli).
Please mouse over
Product Literature References
Mechanical strain modulates extracellular matrix degradation and byproducts in an isoform-specific manner: A. Yeganegi, et al.; Biochim. Biophys. Acta Gen. Subj.
1867, 130286 (2023),
Abstract;
Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing: T.T. Nguyen, et al.; J. Med. Chem.
61, 8825 (2021),
Abstract;
Cell-specific expression of the transcriptional regulator RHAMM provides a timing mechanism that controls appropriate wound re-epithelialization: C. Tolg, et al.; J. Biol. Chem.
295, 5427 (2020),
Abstract;
Full Text
Nucleic acid-induced potentiation of matrix metalloproteinase-9 enzymatic activity: T. Duellman, et al.; Biochem. J.
475, 1597 (2018),
Abstract;
Full Text
Substitutions for arginine at position 780 in triple helical domain of the α1(I) chain alter folding of the type I procollagen molecule and cause osteogenesis imperfecta: E. Makareeva, et al.; PLoS One
13, e0200264 (2018),
Abstract;
Full Text
dCas9-mediated transcriptional activation of tissue inhibitor of metalloproteinases: T. Duellman, et al.; Metalloproteinases Med.
4, 63 (2017),
Abstract;
Full Text
Toxicological effects of NCKU-21, a phenanthrene derivative, on cell growth and migration of A549 and CL1-5 human lung adenocarcinoma cells: H.F. Liao, et al.; PLoS One
12, e28945763 (2017),
Abstract;
Full Text
Active Matrix Metalloprotease-9 Is Associated with the Collagen Capsule Surrounding the Madurella mycetomatis Grain in Mycetoma: K. Geneugelijk, et al.; PLoS Negl. Trop. Dis.
8, e2754 (2014),
Application(s): Gelatin Zymography,
Abstract;
Full Text
Directed evolution of protease beacons that enable sensitive detection of endogenous MT1-MMP activity in tumor cell lines: A. Jabaiah, et al.; Chem. Biol.
18, 392 (2011),
Abstract;
Full Text
IL-1β Is Overexpressed and Aberrantly Regulated in Corticosteroid Nonresponders with Autoimmune Inner Ear Disease: S. Pathak, et al.; J. Immunol.
186, 1870 (2011),
Application(s): Cell Culture,
Abstract;
Full Text
General Literature References
New strategy for antedrug application: development of metalloproteinase inhibitors as antipsoriatic drugs: M. Sawa, et al.; J. Med. Chem.
45, 930 (2002),
Abstract;
Matrix metalloproteinases: they’re not just for matrix anymore!: L.J. McCawley & L.M. Matrisian; Curr. Opin. Cell Biol.
13, 534 (2001),
Abstract;
Structural properties of matrix metalloproteinases: W. Bode, et al.; Cell. Mol. Life Sci.
55, 639 (1999),
Abstract;
Relating matrix metalloproteinase structure to function: why the "hemopexin" domain?: G. Murphy & V. Knauper; Matrix Biol.
15, 511 (1997),
Abstract;
Mechanism of activation of human neutrophil gelatinase B. Discriminating between the role of Ca2+ in activation and catalysis: C.H. Bu & T. Pourmotabbed; J. Biol. Chem.
270, 18563 (1995),
Abstract;
Analysis of the role of the COOH-terminal domain in the activation, proteolytic activity, and tissue inhibitor of metalloproteinase interactions of gelatinase B: J.P. O'Connell, et al.; J. Biol. Chem.
269, 14967 (1994),
Abstract;
Matrix metalloproteinases: a review: H. Birkedal-Hansen, et al.; Crit. Rev. Oral Biol. Med.
4, 197 (1993),
Abstract;
SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages: S.M. Wilhelm, et al.; J. Biol. Chem.
264, 17213 (1989),
Abstract;
Related Products