Product Details
| Alternative Name: | Matrix metalloproteinase 13, Collagenase-3 |
| |
| MW: | 20.4 kDa |
| |
| Source: | Produced in E. coli. Active Matrix Metalloproteinase-13 (MMP-13, collagenase-3) catalytic domain from human cDNA. The enzyme consists of the catalytic domain of human MMP-13 (Tyr104-Asn274, NM_002427) with a C-terminal purification tag. This represents a naturally-occurring active form of MMP-13 which lacks the C-terminal hemopexin domain. MMPs lacking this domain cannot cleave native collagens; however, activity toward other targets such as gelatin, casein, or peptide substrates is unaffected. |
| |
| UniProt ID: | P45452 |
| |
| Formulation: | Liquid. In 50mM TRIS, 5mM CaCl2, 300mM NaCl, 20µM ZnCl2, 0.5% Brij-35, and 30% glycerol. |
| |
| Purity: | ≥95% (SDS-PAGE) |
| |
| Purity Detail: | Purified by multi-step chromatography. |
| |
| Activity: | Preincubation of MMP-13 catalytic domain at 13nM with the broad-spectrum inhibitor GM6001 (Prod. No. BML-EI300) at 20nM for 1 hour completely inhibits enzymatic activity. |
| |
| Specific Activity: | ≥2000 pmol/min/µg at 37°C using the colorimetric thiopeptolide Ac-Pro-Leu-Gly-S-Leu-Leu-Gly-OEt (100 µM; Prod. No. BML-P125) as substrate. |
| |
| Application Notes: | Useful tool to study enzyme kinetics, cleave target substrates, and screen for inhibitors. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
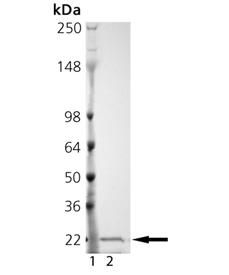
SDS-PAGE Analysis: Lane1: MW Marker; Lane 2: 2µg of purified MMP-13 (catalytic domain) (human), (recombinant).
Please mouse over
Product Literature References
Development of Selective ADAMTS-5 Peptide Substrates to Monitor Proteinase Activity: M.F. Fowkes, et al.; J. Med. Chem.
66, 3522 (2023),
Abstract;
HIV‐1 Tat promotes astrocytic release of CCL2 through MMP/PAR‐1 signaling: P.L. Bozzelli, et al.; Glia
67, 1719 (2019),
Abstract;
Enzymatic, physicochemical and biological properties of MMP-sensitive alginate hydrogels: K.B. Fonseca, et al.; Soft Matter 9, 3283 (2013),
Development and validation of novel enzyme activity methods to assess inhibition of matrix metalloproteinases (MMPs) in human serum by antibodies against enzyme therapeutics: T.J. Edkins, et al.; J. Pharm. Biomed. Anal.
70, 408 (2012),
Abstract;
Directed evolution of protease beacons that enable sensitive detection of endogenous MT1-MMP activity in tumor cell lines: A. Jabaiah, et al.; Chem. Biol.
18, 392 (2011),
Abstract;
Full Text
The in vitro resistance of IgG2 to proteolytic attack concurs with a comparative paucity of autoantibodies against peptide analogs of the IgG2 hinge: R.J. Brezski, et al.; Mabs
3, 558 (2011),
Abstract;
Full Text
The effect of a hydroxamic acid-containing polymer on active matrix metalloproteinases: G.A. Skarja, et al.; Biomaterials
30, 1890 (2009),
Abstract;
Related Products










