Product Details
| Alternative Name: | Matrix metalloproteinase 1, Interstitial collagenase, Fibroblast collagenase |
| |
| MW: | ~19.9 kDa |
| |
| Source: | Produced in E. coli. Active Matrix Metalloproteinase-1 (MMP-1, interstitial collagenase, fibroblast collagenase) catalytic domain from human cDNA. The enzyme consists of the catalytic domain of human MMP-1 (Phe100-Gln268) with a C-terminal purification tag. |
| |
| UniProt ID: | P03956 |
| |
| Gene/Protein Identifier: | NM_002421 (RefSeq) |
| |
| Formulation: | Liquid. In 50mM TRIS, pH 7.5, containing 5mM calcium chloride, 300mM sodium chloride, 20µM zinc chloride, 0.5% Brij-35, and 30% glycerol. |
| |
| Purity: | ≥95% (SDS-PAGE) |
| |
| Purity Detail: | Purified by multi-step chromatography. |
| |
| Activity: | Preincubation of MMP-1 catalytic domain at 0.7 nM with the broad-spectrum inhibitor GM6001 (Prod. No. BML-EI300) at 20nM for 1 hour completely inhibits enzymatic activity. |
| |
| Specific Activity: | ≥5000 pmol/min/µg at 37°C using the colorimetric thiopeptolide Ac-Pro-Leu-Gly-S-Leu-Leu-Gly-OEt (100 µM; Prod. No. BML-P125) as substrate. |
| |
| Application Notes: | Useful tool to study enzyme kinetics, cleave target substrates, and screen for inhibitors. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Use/Stability: | The enzyme is stable on ice for at least several hours. However, it is recommended that thawing and dilution of the enzyme be done within as short a time as possible before start of the assay. Note: When stored under the recommended conditions, this enzyme is stable at the concentration supplied, in its current storage buffer. Procedures such as dilution of the enzyme followed by refreezing could lead to loss of activity. |
| |
| Handling: | Avoid freeze/thaw cycles. After opening, prepare aliquots and store at -80°C. |
| |
| Scientific Background: | In an SDS-PAGE gel, the enzyme runs as a doublet (<20 kDa). The higher band represents the polypeptide described above, while spontaneous cleavage of the tag results in the lower band. Both species possess identical enzymatic activities. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
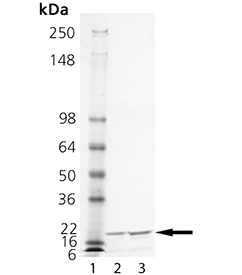
SDS-PAGE Analysis: Lane 1: MW Marker, Lane 2: 1 µg, Lane 3: 2 µg MMP-1.
Please mouse over
Product Literature References
Mechanical strain modulates extracellular matrix degradation and byproducts in an isoform-specific manner: A. Yeganegi, et al.; Biochim. Biophys. Acta Gen. Subj.
1867, 130286 (2023),
Abstract;
Validation of Matrix Metalloproteinase-9 (MMP-9) as a Novel Target for Treatment of Diabetic Foot Ulcers in Humans and Discovery of a Potent and Selective Small-Molecule MMP-9 Inhibitor That Accelerates Healing: T.T. Nguyen, et al.; J. Med. Chem.
61, 8825 (2021),
Abstract;
Structural and functional insights into the interaction of sulfated glycosaminoglycans with tissue inhibitor of metalloproteinase-3 - A possible regulatory role on extracellular matrix homeostasis: S. Rother, et al.; Acta Biomater.
45, 143 (2016),
Abstract;
Enzymatic, physicochemical and biological properties of MMP-sensitive alginate hydrogels: K.B. Fonseca, et al.; Soft Matter 9, 3283 (2013),
Development and validation of novel enzyme activity methods to assess inhibition of matrix metalloproteinases (MMPs) in human serum by antibodies against enzyme therapeutics: T.J. Edkins, et al.; J. Pharm. Biomed. Anal.
70, 408 (2012),
Abstract;
Directed evolution of protease beacons that enable sensitive detection of endogenous MT1-MMP activity in tumor cell lines: A. Jabaiah, et al.; Chem. Biol.
18, 392 (2011),
Abstract;
Full Text
Synthesis, kinetic characterization and metabolism of diastereomeric 2-(1-(4-phenoxyphenylsulfonyl)ethyl)thiiranes as potent gelatinase and MT1-MMP inhibitors: M. Gooyit, et al.; Chem. Biol. Drug Des.
74, 535 (2009),
Abstract;
Related Products