Product Details
| Alternative Name: | Mch2 |
| |
| MW: | 18 + 11 kDa subunits |
| |
| Source: | Produced in E. coli. |
| |
| UniProt ID: | P55212 |
| |
| Formulation: | Liquid. In 50mM HEPES, pH 7.4, containing 100mM sodium chloride, 0.5% CHAPS, 1mM EDTA, 10% glycerol and 10mM DTT. |
| |
| Purity: | ≥80% (SDS-PAGE) |
| |
| Activity: | 100 U/µl |
| |
| Specific Activity: | One U=1 pmol/min at 30°C using Ac-VEID-pNA (200mM; Prod. No. BML-P437) as substrate. |
| |
| Application Notes: | Useful tool to study enzyme regulation and kinetics, cleave target substrates, screen for inhibitors. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Use/Stability: | After initial defrost, aliquot product into individual tubes and refreeze the remaining, unused enzyme quickly by snap-freezing in a dry/ice ethanol bath or liquid nitrogen, if possible. |
| |
| Handling: | Avoid freeze/thaw cycles. |
| |
| Scientific Background: | Effector caspase responsible for the cleavage of nuclear lamins in apoptosis. Caspase-6 cleavages of various other protein targets have been implicated in the pathogenesis of neurodegenerative diseases, including Alzheimer’s disease (b-amyloid precursor protein), Huntington’s disease (huntingtin). |
| |
| Regulatory Status: | RUO - Research Use Only |
| |

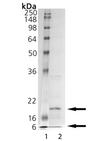
SDS-PAGE Analysis - Lane 1: MW Marker, Lane 2: 1 µg Caspase-6.
Please mouse over
Product Literature References
Characterization of calpain and caspase-6 generated glial fibrillary acidic protein breakdown products follow ing traumatic brain injury and astroglial cell injury: Z. Yang, et al.; Int. J. Mol. Sci.
23, 8960 (2022),
Abstract;
GSDMEa-mediated pyroptosis is bi-directionally regulated by caspase and required for effective bacterial clearance in teleost: H. Xu, et al.; Cell Death Dis.
13, 491 (2022),
Abstract;
Huntingtin structure is orchestrated by HAP40 and shows a polyglutamine expansion-specific interaction with exon 1: R.J. Harding, et al.; Commun. Biol.
4, 1374 (2021),
Abstract;
Activation of caspase-6 is promoted by a mutant huntingtin fragment and blocked by an allosteric inhibitor compound: D.E. Ehrnhoefer, et al.; Cell Chem. Biol.
26, 1295 (2019),
Abstract;
Full Text
Quantification of Total and Mutant Huntingtin Protein Levels in Biospecimens Using a Novel alphaLISA Assay: B. Baldo, et al.; eNeuro
5, ENEURO.0234-18.2018 (2018),
Abstract;
Full Text
Laquinimod decreases Bax expression and reduces caspase-6 activation in neurons: D. Ehrnhoefer, et al.; Exp. Neurol.
283A, 121 (2016),
Application(s): Caspase-6 inhibition (in vitro),
Abstract;
Caspase-6 activity in a BACHD mouse modulates steady-state levels of mutant huntingtin protein but is not necessary for production of a 586 amino acid proteolytic fragment: J. Gafni, et al.; J. Neurosci
32, 7454 (2012),
Abstract;
Induction of the C-terminal proteolytic cleavage of AβPP by statins: O. Descamps, et al.; J. Alzheimers Dis.
25, 51 (2011),
Abstract;
Identification and Evaluation of Novel Small Molecule Pan-Caspase Inhibitors in Huntington’s Disease Models: M.J. Leyva, et al.; Chem. Biol.
17, 1189 (2010),
Abstract;
General Literature References
Caspase-mediated cleavage of DNA topoisomerase I at unconventional sites during apoptosis: K. Samejima, et al.; J. Biol. Chem.
274, 4335 (1999),
Abstract;
Biochemical characteristics of caspases-3, -6, -7, and -8: H.R. Stennicke & G.S. Salvesen; J. Biol. Chem.
272, 25719 (1997),
Abstract;
Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells: L. Faleiro, et al.; EMBO J.
16, 2271 (1997),
Abstract;
Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis: A. Takahashi, et al.; PNAS
93, 8395 (1996),
Abstract;
The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A: K. Orth, et al.; J. Biol. Chem.
271, 16443 (1996),
Abstract;
Mch2, a new member of the apoptotic Ced-3/Ice cysteine protease gene family: T. Fernandes-Alnemri, et al.; Cancer Res
55, 2737 (1995),
Abstract;
Related Products