Replaces Prod. #: ALX-380-047
Nucleoside antibiotic. Inhibits protein N-glycosylation by blocking the transfer of N-acetylglucosamine 1-phosphate to dolichol monophosphate. Induces ER-stress following the inhibition of N-linked glycosylation. Arrests cell cycle in late G1. Unstable at acidic pH.
Product Details
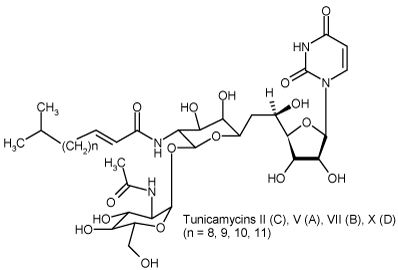
| Formula: | C38H62N4O16 (Tun B) |
| |
| MW: | Avg. 840.0 |
| |
| Source: | Isolated from Streptomyces lysosuperficus. |
| |
| CAS: | 11089-65-9 |
| |
| MI: | 14: 9819 |
| |
| RTECS: | YO7980200 |
| |
| Purity: | ≥99% (TLC) |
| |
| Purity Detail: | Mixture of tunicamycin A, B, C and D. |
| |
| Appearance: | White solid to tan solid. |
| |
| Solubility: | Soluble in DMSO (>10mg/ml) or methanol (5mg/ml; warm). |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | -20°C |
| |
| Use/Stability: | Stable for at least 1 year after receipt when stored, as supplied, at -20°C. Stock solutions are stable for up to 3 months at -20°C. |
| |
| Technical Info/Product Notes: | Replacement for ADI-908-297. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
ER stress-induced cell death proceeds independently of the TRAIL-R2 signaling axis in pancreatic β cells: C. Hagenlocher, et al.; Cell Death Discov.
8, 34 (2022),
Abstract;
CHOP favors endoplasmic reticulum stress-induced apoptosis in hepatocellular carcinoma cells via inhibition of autophagy: Y. Lei, et al.; PLoS One
12, e0183680 (2017),
Abstract;
Full Text
Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells: C.A. Tang, et al.; Cancer Res.
76, 2137 (2016),
Application(s): Stimulated cells,
Abstract;
Full Text
An inhibitor of HIV-1 protease modulates constitutive eIF2α dephosphorylation to trigger a specific integrated stress response: A. De Gassart, et al.; PNAS
113, E117 (2016),
Application(s): Cell culture,
Abstract;
Full Text
Anti-Inflammatory Activity of Cyanobacterial Serine Protease Inhibitors Aeruginosin 828A and Cyanopeptolin 1020 in Human Hepatoma Cell Line Huh7 and Effects in Zebrafish (Danio rerio): S. Faltermann, et al.; Toxins (Basel)
8, 219 (2016),
Application(s): EROD assay in Huh7 Cells; Zebrafishliver organ culture and gene expression analysis,
Abstract;
Full Text
Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway: K. Shenderov, et al.; J. Immunol.
192, 2029 (2014),
Abstract;
Glycosylation modulates TRAIL-R1/death receptor 4 protein: different regulations of two pro-apoptotic receptors for TRAIL by tunicamycin: T. Yoshida, et al.; Oncol. Rep.
18, 1239 (2007),
Abstract;
Tunicamycin sensitizes human melanoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by up-regulation of TRAIL-R2 via the unfolded protein response: C.C. Jiang, et al.; Cancer Res.
67, 5880 (2007),
Abstract;
Novel potential of tunicamycin as an activator of the aryl hydrocarbon receptor -- dioxin responsive element signaling pathway: K. Horikawa, et al.; FEBS Lett.
580, 3721 (2006),
Abstract;
Tunicamycin enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human prostate cancer cells: T. Shiraishi, et al.; Cancer Res.
65, 6364 (2005),
Abstract;
Tunicamycin inhibits NMDA and AMPA receptor responses independently of N-glycosylation: K. Maruo, et al.; Brain Res.
977, 294 (2003),
Abstract;
Tunicamycin inhibits the expression of functional thrombin receptors on human T-lymphoblastoid cells: A. Tordai, et al.; BBRC
206, 857 (1995),
Abstract;
Role of N-glycosylation in the structure and function of the methotrexate membrane transporter from CCRF-CEM human lymphoblastic leukemia cells: L.H. Matherly & S.M. Angeles; Biochem. Pharmacol.
47, 1094 (1994),
Abstract;
Isoprenoids and astroglial cell cycling: diminished mevalonate availability and inhibition of dolichol-linked glycoprotein synthesis arrest cycling through distinct mechanisms: T.J. Langan & M.C. Slater; J. Cell Physiol.
149, 284 (1991),
Abstract;
Dolichol-linked glycoprotein synthesis in G1 is necessary for DNA synthesis in synchronized primary cultures of cerebral glia: S. Ishii & J.J. Volpe; J. Neurochem.
49, 1606 (1987),
Abstract;
Relationship of the structure and biological activity of the natural homologues of tunicamycin: D. Duksin & W.C. Mahoney; J. Biol. Chem.
257, 3105 (1982),
Abstract;
Full Text
Isolation and structures of components of Tunicamycin: T. Ito, et al.; Agric. Biol. Chem. 44, 695 (1980),
Separation of tunicamycin homologues by reversed-phase high-performance liquid chromatography: W.C. Mahoney & D. Duksin; J. Chromatogr.
198, 506 (1980),
Abstract;
Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin: A. Takatsuki, et al.; J. Antibiot. (Tokyo)
24, 215 (1971),
Abstract;