-
Useful for inhibitor screening or characterizing enzyme kinetics
-
Includes optimal substrate selected from a panel of acetylated sites in p53 and histones
-
Supplied with enough recombinant enzyme for 96 assays (1 x 96-well plate)
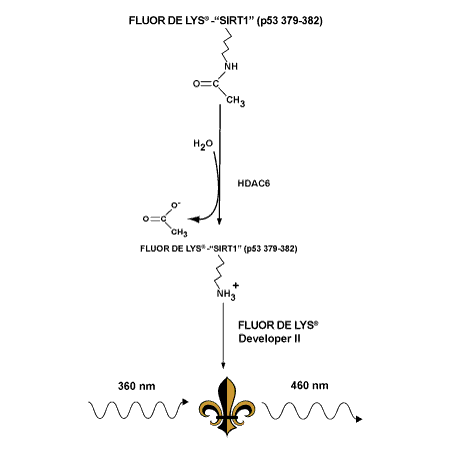
A FLUOR DE LYS® fluorescent assay system. The HDAC6 Fluorescent Activity Assay/Drug Discovery Kit is a complete assay system designed to measure the lysyl deacetylase activity of the recombinant human HDAC6 included in the kit. The kit is ideal for chemical library screening for candidate inhibitors or activators or kinetic assay of the enzyme under varying conditions. The FLUOR DE LYS® HDAC6 assay is based on the FLUOR DE LYS® Substrate and FLUOR DE LYS® Developer combination. The assay procedure has two steps. First, the FLUOR DE LYS® Substrate, which comprises an acetylated lysine side chain, is incubated with HDAC6. Deacetylation of the substrate sensitizes the substrate so that, in the second step, treatment with the FLUOR DE LYS®Developer produces a fluorophore.
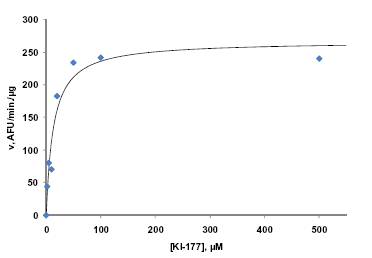
Figure 2: Kinetics of FLUOR DE LYS® SIRT1 (Prod. No. BML-KI177) substrate deacetylation by HDAC6. Method: HDAC6 enzyme (500 ng/well) was incubated (37°C) with indicated concentrations of substrate. Reactions were stopped after 60min. with FLUOR DE LYS® Developer II and fluorescence was measured. Points are the mean of two independent determinations, each comprising three replicates. Line is a non-linear least squares fit of the data to the Michaelis-Menten equation. The best-fit Km was 13.0µM and the Vmax 267 AFU/min/µg.
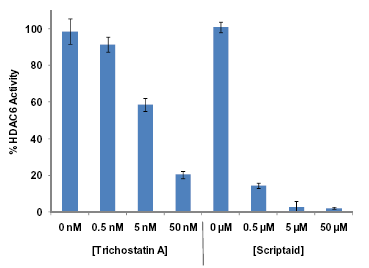
Figure 3: Trichostatin A and Scriptaid (Prod. No. BML-GR326) inhibition of HDAC6 determined by FLUOR DE LYS®-SIRT1 (Prod. No. BML-KI177) substrate deacetylation. Method: HDAC6 enzyme (400 ng/well) was incubated (37°C) with 12µM substrate at the indicated concentrations of Trichostatin A and Scriptaid. Reactions were stopped after 60min. with FLUOR DE LYS® Developer II and the fluorescence was measured.
Please mouse over
Product Details
| Alternative Name: | Histone deacetylase 6 fluorescent assay kit |
| |
| Applications: | Fluorescent detection, HTS
Activity assay
|
| |
| Use/Stability: | Store all components except the microplate at -80°C for the highest stability. The HDAC6 Enzyme (Prod. No. BML-SE508), must be handled with particular care in order to retain maximum enzymatic activity. Defrost it quickly in a RT water bath or by rubbing between fingers, then immediately store on an ice bath. The remaining unused enzyme should be refrozen quickly, by placing at -80°C. If possible, snap freeze in liquid nitrogen or a dry ice/ethanol bath. To minimize the number of freeze/thaw cycles, aliquot the enzyme into separate tubes and store at -80°C. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Contents: | HDAC6 (Histone Deacetylase 6) (human, recombinant) (Prod. No. BML-SE508)
(50 µg; 10mM TRIS, pH 7.5, 100mM NaCl, 3mM MgCl2, 10% glycerol)
Storage: -80°C, avoid freeze/thaw cycles!
FLUOR DE LYS® SIRT1, Deacetylase Substrate (Prod. No. BML-KI177)
(100µl; 5mM solution in 50mM TRIS/Cl, pH 8.0, 137mM NaCl, 2.7mM KCl, 1mM MgCl2)
Storage: -80°C
FLUOR DE LYS® Developer II Concentrate (5x) (Prod. No. BML-KI176)
(5 x 250 µl; 5x Stock Solution; Dilute in Assay Buffer before use
Storage: -80°C
Trichostatin A (HDAC Inhibitor) (Prod. No. BML-GR309-9090)
(100 µl; 0.2mM in DMSO)
Storage: -80°C
FLUOR DE LYS® Deacetylated Standard (Prod. No. BML-KI142)
(30 µl; 10mM in DMSO)
Storage: -80°C
HDAC Assay Buffer II (Prod. No. BML-KI422)
(20ml; 50mM TRIS/Cl, pH 8.0, 137mM NaCl, 2.7mM KCl, 1mM MgCl2, 1mg/ml BSA)
Storage: -20°C
HDAC Assay Buffer (Prod. No. BML-KI143)
(20ml; 50mM TRIS/Cl, pH 8.0, 137mM NaCl, 2.7mM KCl, 1mM MgCl2)
Storage: -20°C
1/2 volume microplate (Prod. No. BML-KI101)
Storage: Ambient
1/2 volume white NBS microplate (Prod. No. BML-KI584)
Storage: Ambient |
| |
| Scientific Background: | HDAC6 is a class II HDAC, a group defined by its member’s homology to the yeast HDAC. HDAC6 falls into the class IIb subclass, along with HDAC10, but is unique among human HDACs in that it contains two full deacetylase domains. Primarily located to cytoplasm, HDAC6 is a tubulin deacetylase. Other HDAC6 substrates include Hsp90, cortactin and peroxiredoxins. Through these deacetylase activities and its ubiquitin and other binding activities, HDAC6 plays key regulatory roles in cell motility, protein folding, the ubiquitin-proteasome pathway, the aggresome pathway and autophagy, suggesting a central function in the coordination of cellular stress responses. HDAC6 is required for efficient oncogenic tumorigenesis. The mechanisms underlying this requirement may include HDAC6-mediated promotion of invasive cell motility, resistance to anoikis, removal of toxic misfolded proteins, inhibition of Hsp90 stabilization of metastasis suppressors and promotion of angiogenesis. HDAC6 null-mice, while outwardly normal, have increased resistance to chemical carcinogenesis. It is thus reasonable to suppose that HDAC6 inhibition may be a key factor in the anti-cancer effects of pan-HDAC inhibitors and that selective HDAC6 inhibitors may have the potential for greater effectiveness and reduced side-effects. |
| |
| UniProt ID: | Q9UBN7 |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
Novel Multitarget Hydroxamic Acids with a Natural Origin CAP Group against Alzheimer’s Disease: Synthesis, Docking and Biological Evaluation: M. Neganova, et al.; Pharmaceutics
13, 1893 (2021),
Abstract;
Synthesis and Biological Evaluation of HDAC Inhibitors With a Novel Zinc Binding Group: J. He, et al.; Front. Chem.
8, 256 (2020),
Abstract;
Full Text
Aberrant GlyRS-HDAC6 interaction linked to axonal transport deficits in Charcot-Marie-Tooth neuropathy: Z. Mo, et al.; Nat. Commun.
9, 1007 (2018),
Abstract;
Full Text
Design, Synthesis and Biological Evaluation of a Phenyl Butyric Acid Derivative, N-(4-chlorophenyl)-4-phenylbutanamide: A HDAC6 Inhibitor with Anti-proliferative Activity on Cervix Cancer and Leukemia Cells: R.A. Rodriiguez-Fonseca, et al.; Anticancer Agents Med. Chem.
17, 1441 (2017),
Abstract;
Computer-aided identification of new histone deacetylase 6 selective inhibitor with anti-sepsis activity: J. Yoo, et al.; Eur. J. Med. Chem.
116, 126 (2016),
Abstract;
Hybrid Enzalutamide Derivatives with Histone Deacetylase Inhibitor Activity Decrease Heat Shock Protein 90 and Androgen Receptor Levels and Inhibit Viability in Enzalutamide-Resistant C4-2 Prostate Cancer Cells: R. Rosati, et al.; Mol. Pharmacol.
90, 225 (2016),
Abstract;
Full Text
NBM-T-BBX-OS01, Semisynthesized from Osthole, Induced G1 Growth Arrest through HDAC6 Inhibition in Lung Cancer Cells: J.T. Pai, et al.; Molecules
20, 8000 (2015),
Application(s): Fluorescence Microscopy,
Abstract;
Full Text
Targeting the invasive phenotype of cisplatin-resistant Non-Small Cell Lung Cancer cells by a novel histone deacetylase inhibitor: V. Zuco, et al.; Biochem. Pharmacol.
94, 79 (2015),
Application(s): Assay,
Abstract;
Design, synthesis, and biological activity of NCC149 derivatives as histone deacetylase 8-selective inhibitors: T. Suzuki, et al.; ChemMedChem.
9, 657 (2014),
Abstract;
Identification of highly selective and potent histone deacetylase 3 inhibitors using click chemistry-based combinatorial fragment assembly: T. Suzuki, et al.; PLoS One
8, e68669 (2013),
Abstract;
Full Text
Association of estrogen receptor alpha and histone deacetylase 6 causes rapid deacetylation of tubulin in breast cancer cells: K. Azuma, et al.; Cancer Res.
69, 2935 (2009),
Abstract;
Full Text
HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation: R.B. Parmigiani, et al.; PNAS
105, 9633 (2008),
Abstract;
Full Text
HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions: A. Valenzuela-Fernandez, et al.; Trends Cell Biol.
18, 291 (2008),
Abstract;
Role of the aggresome pathway in cancer: targeting histone deacetylase 6-dependent protein degradation: A. Rodriguez-Gonzalez, et al.; Cancer Res.
68, 2557 (2008),
Abstract;
Full Text
The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis: Y.S. Lee, et al.; Cancer Res.
68 , 7561 (2008),
Abstract;
Full Text
Design, synthesis, structure--selectivity relationship, and effect on human cancer cells of a novel series of histone deacetylase 6-selective inhibitors: Y. Itoh, et al.; J. Med. Chem.
50, 5425 (2007),
Abstract;
HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions: A.D. Tran, et al.; J. Cell Sci.
120, 1469 (2007),
Abstract;
Full Text
HDAC6 modulates cell motility by altering the acetylation level of cortactin: X. Zhang, et al.; Mol. Cell
27, 197 (2007),
Abstract;
Full Text
HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination: C. Boyault, et al.; Oncogene
26, 5468 (2007),
Abstract;
Breast cancer metastasis suppressor 1 (BRMS1) is stabilized by the Hsp90 chaperone: D.R. Hurst, et al.; Biochem. Biophys. Res. Commun.
348, 1429 (2006),
Abstract;
Full Text
Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1 alpha: D.Z. Qian, et al.; Cancer Res.
66, 8814 (2006),
Abstract;
Full Text
Two catalytic domains are required for protein deacetylation: Y. Zhang, et al.; J. Biol. Chem.
281, 2401 (2006),
Abstract;
Full Text
HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin: A. Iwata, et al.; J. Biol. Chem.
280, 40282 (2005),
Abstract;
Full Text
HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor: J.J. Kovacs, et al.; Mol. Cell
18, 601 (2005),
Abstract;
Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors: P. Bali, et al.; J. Biol. Chem.
280, 26729 (2005),
Abstract;
Full Text
Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer: S. Saji, et al.; Oncogene
24, 4531 (2005),
Abstract;
Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis.: I.V. Gregoretti, et al.; J. Mol. Biol.
338, 17 (2004),
Abstract;
Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation: S.J. Haggarty, et al.; PNAS
100, 4389 (2003),
Abstract;
Full Text
HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo: Y. Zhang, et al.; EMBO J.
22, 1168 (2003),
Abstract;
Full Text
The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress: Y. Kawaguchi, et al.; Cell
115, 727 (2003),
Abstract;
HDAC6 is a microtubule-associated deacetylase: C. Hubbert, et al.; Nature
417, 455 (2002),
Abstract;
In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation: A. Matsuyama, et al.; EMBO J.
21, 6820 (2002),
Abstract;
Full Text
Three proteins define a class of human histone deacetylases related to yeast Hda1p: C. Grozinger, et al.; PNAS
96, 4868 (1999),
Abstract;
Full Text
A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p: J. Taunton, et al.; Science
272, 408 (1996),
Abstract;