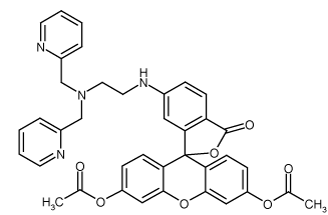
Cell permeable derivative of ZnAF-2 (Prod. No. ALX-620-072). Fluorescent reagent (Ex(max): 492nm; Em(max): 514nm) for the detection of low concentration of zinc ion due to its strong affinity to zinc ion (dissociation constant: 2.7nM). The sample zinc ion can be specifically detected. Low background fluorescense supersensitizes the visualization for in vivo sample zinc ion.
Product Details
| Alternative Name: | 6-[N-[N',N'-bis(2-Pyridinylmethyl)-2-aminoethyl]amino-3',6'-bis(acetyloxy)-spiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one |
| |
| Formula: | C38H32N4O7 |
| |
| MW: | 656.7 |
| |
| CAS: | 357339-96-9 |
| |
| Concentration: | ~5mM |
| |
| Formulation: | Dissolved in 0.3ml DMSO. |
| |
| Appearance: | Liquid. |
| |
| Shipping: | Blue Ice |
| |
| Long Term Storage: | -20°C |
| |
| Use/Stability: | Prepare 500-5’000-fold dilution (~10-1µM) in HEPES buffer (0.1M phosphate, pH 7.4) immediately before use. BSA, phenol red and amines may affect the fluorescence and must be used with caution. Do not store the dilutions. |
| |
| Handling: | Protect from light. Keep under inert gas. After opening, prepare aliquots and store at -20°C. |
| |
| Scientific Background: | Zinc (Zn) is the second most abundant transition metal in the body ant it is essential as catalytic, structural and regulatory ion. Zinc ions are involved in homeostasis, immune responses, oxidative stress, apoptosis and aging. Zinc has been proposed to function as a conventional neurotransmitter for the presynaptic neuron and as a transmembrane signal to traverse the postsynaptic neuron. Aberrant zinc metabolism is associated with many neurological diseases including Alzheimer’s disease, Parkinson’s disease and epilepsy. The most suitable technique for in vivo monitoring of zinc has been proven to be fluroescent imaging. |
| |
| Protocol: | Zn2+ imaging with macrophage (RAW 264.7) (Figure 2)
Culture: 37°C, 5% CO2, 95% air, Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 1% penicillin and streptomycin.
Washing: Washed twice with PBS.
Staining: Incubation with PBS containing 10µm ZnAF-2 DA at 37°C for 0.5 hours.
Measure: Measured using a fluorescence microscope.
Result: An increase of the fluorescence intensity was observed within intercellular region (1-3) as the concentration of zinc was increased by adding 5µM of pyrithione and 50µM of zinc sulfate into extracellular fluid at 5 min after start of measurement. Few changes appeared within extracellular region (4). After 20 min, the fluorescence intensity decreased as the concentration of zinc ion in cells was decreased by the addition of TPEN (Prod. No. ALX-450-011) to the extracellular fluid.
Imaging of rat hippocampal slides (Figure 3)
Preparation of slides: Whole brain including hippocampus was removed under Ringer's solution and cut into 300µm thick slides. The slides were put into Ringer's solution at 30°C for 30 min.
Staining: The slides were put into Ringer's solution containing 10µM ZnAF-2 DA and incubated at room temperature for 1.5 hours.
Washing: Slides were washed for 1.5 hours.
Measure: Slides were placed in the chamber which was set on a stage of the fluorescence microscope. Ringer's solution was perfused in the chamber at 2.5ml/min. The inside temperature of the chamber was 33-34°C. The Ringer's solution was consistently being bubbled with 5% CO2, 95% air.
Changes in the imaging fluorescence of the hippocampal slides stained with ZnAF-2 DA with time (Figure 4)
Changes in the fluorescence intensity (the starting intensity is 1 and the changes of the ratio are showed in artifical colors) as the ischemic stimulation, in which concentrations of glucose and oxygen in the perfusion liquid were decreased, was given. Intensification of the alternative fluorescence was showed in CA1 (1 and 2). It is believed that the intensification physiologically implied the involvement in the alternative death of nerve cells during cerebral ischemia (caused in CA1). |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
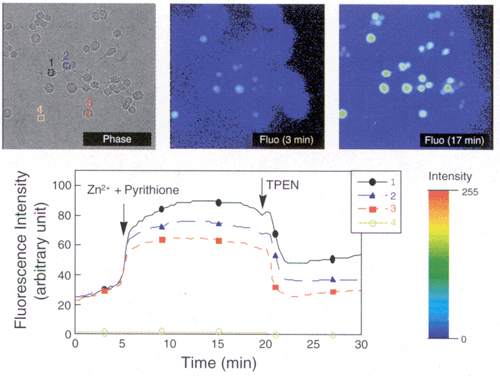
Figure 2: Zn2+ imaging with macrophage (RAW 264.7).
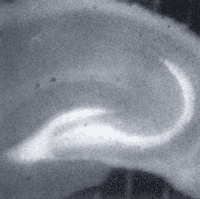
Figure 3: Rat hippocampal slices stained with ZnAF-2 DA.

Figure 4: Changes in the imaging fluorescence of the hippocampal slices stained with ZnAF-2 DA over time.
Please mouse over
Product Literature References
Expression profile of the zinc transporter ZnT3 in taste cells of rat circumvallate papillae and its role in zinc release, a potential mechanism for taste stimulation: K. Nishida, et al.; Eur. J. Histochem.
66, 3534 (2022),
Abstract;
Zinc-mediated attenuation of hippocampal mossy fiber long-term potentiation induced by forskolin: M. Ando, et al.; Neurochem. Int.
57, 608 (2010),
Abstract;
Zinc is an essential trace element for spermatogenesis: S. Yamaguchi, et al.; PNAS U.S.A.
106, 10859 (2009),
Abstract;
Full Text
Negative modulation of presynaptic activity by zinc released from Schaffer collaterals: A. Takeda, et al.; J. Neurosci
85, 3666 (2007),
Abstract;
Influence of location of a fluorescent zinc probe in brain slices on its response to synaptic activation: A.R. Kay & K. Toth; J. Neurophysiol.
95, 1949 (2006),
Abstract;
Full Text
Improvement and biological applications of fluorescent probes for zinc, ZnAFs: T. Hirano, et al.; JACS
124, 6555 (2002),
Abstract;
Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits: S. Ueno, et al.; J. Cell Biol.
158, 215 (2002),
Abstract;
Full Text
Highly Zinc-Selective Fluorescent Sensor Molecules Suitable for Biological Applications: T. Hirano, et al.; JACS
122, 12399 (2000),
Full Text
General Literature References
Mechanism and regulation of cellular zinc transport: I. Sekler, et al.; Mol. Med.
13, 337 (2007),
Abstract;
Full Text
Sticky fingers: zinc-fingers as protein-recognition motifs: R. Gamsjaeger, et al.; TIBS
32, 63 (2007),
Abstract;
Zinc: a multipurpose trace element: M. Stefanidou, et al.; Arch. Toxicol.
80, 1 (2006),
Abstract;
Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors: P. Jiang & Z. Guo; Coord. Chem. Rev. 248, 205 (2004),
Related Products