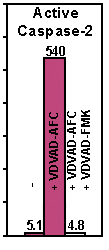Product Details
| Source: | Produced in E. coli. Contains an N-terminal His-tag. |
| |
| EC: | 3.4.22.36 |
| |
| UniProt ID: | P42575 |
| |
| Formulation: | Lyophilized. |
| |
| Purity: | ≥95% (SDS-PAGE) |
| |
| Specific Activity: | 10,000 - 13,000 units/mg. One unit of recombinant caspase-2 is the enzyme activity that cleaves 1 nmol of caspase substrate VDVAD-pNA (pNA: p-nitroaniline) per hour at 37ºC in a reaction solution containing 50 mM HEPES, pH 7.2, 50 mM NaCl, 0.1% CHAPS, 10 mM EDTA, 5% Glycerol, and 10 mM DTT. |
| |
| Application Notes: | Useful in screening caspase inhibitors, studying enzyme regulation and kinetics, determining specificity of capase substrates, or as positive control in caspase activity assays. We recommend using 1 unit per assay for analyzing caspase activity. For a complete caspase-2 assay protocol, please refer to Caspase-2 Fluorometric Assay Kit (Prod. No. ALX-850-214). |
| |
| Reconstitution: | Reconstitute to 1U/µl with PBS containing 15% glycerol. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Handling: | After reconstitution, prepare aliquots and store at -80°C. |
| |
| Scientific Background: | Caspase-2 is a member of the interleukin-1β converting enzyme (ICE) family of cysteine proteases. Similar as other caspases, caspase-2 also exists in cells as an inactive proenzyme. During apoptosis, procaspase-2 is processed at aspartate residues by self-proteolysis and/or cleavage by upstream caspases. The processed form of caspase-2 consists of large (19kDa) and small (12kDa) subunits which associate to form the active enzyme. The expressed caspase-2 spontaneously undergoes auto-processing to yield the subunits characteristic of the native enzyme. The active recombinant caspase-2 is routinely tested for its ability to enzymatically cleave the caspase-2 colorimetric substrate Ac-VDVAD-pNA (Prod. No. ALX-260-059). The signal can be detected by spectrophotometry. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
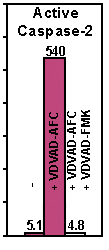
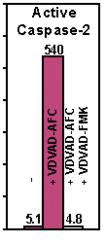
Figure: Active human caspase was expressed in E. coli and purified. The activity of recombinant caspase-2 was determined by cleaving AFC conjugates of VDVAD. The cleavage activity was effectively inhibited by the corresponding peptide inhibitor as indicated.
Please mouse over
Product Literature References
GSDMEa-mediated pyroptosis is bi-directionally regulated by caspase and required for effective bacterial clearance in teleost: H. Xu, et al.; Cell Death Dis.
13, 491 (2022),
Abstract;
Identification and Evaluation of Novel Small Molecule Pan-Caspase Inhibitors in Huntington’s Disease Models: M.J. Leyva, et al.; Chem. Biol.
17, 1189 (2010),
Abstract;
Related Products