Product Details
| Alternative Name: | Heat shock cognate 71 kDa protein, Heat shock 70 kDa protein 8, HSPA8 |
| |
| MW: | ~73kDa |
| |
| Source: | Produced in E. coli. |
| |
| UniProt ID: | P19120 |
| |
| Formulation: | Liquid. In 30mM TRIS-HCl, pH 7.5, containing 1.0mM EDTA, 2.0mM DTT, and 300mM sodium chloride. |
| |
| Purity: | ≥90% (SDS-PAGE; Western blot) |
| |
| Purity Detail: | Purified by multi-step chromatography. |
| |
| Applications: | WB
Activity assay
|
| |
| Application Notes: | ATPase activity assay (positive). Western blot control. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Scientific Background: | The Hsp70 family of heat shock proteins contains multiple homologs ranging in size from 66-78 kDa, and are the eukaryotic equivalents of the bacterial DnaK. The most studied Hsp70 members include the cytosolic stress-induced Hsp70 (Hsp72), the constitutive cytosolic Hsc70 (Hsp73), and the ER-localized BiP (Grp78). Hsp70 family members contain highly conserved N-terminal ATP-ase and C-terminal protein binding domains. Binding of peptide to Hsp70 is assisted by Hsp40, and stimulates the inherent ATPase activity of Hsp70, facilitating ATP hydrolysis and enhanced peptide binding. Hsp70 nucleotide exchange and substrate binding coordinates the folding of newly synthesized proteins, the re-folding of misfolded or denatured proteins, coordinates trafficking of proteins across cellular membranes, inhibits protein aggregation, and targets the degradation of proteins via the proteasomal pathway. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
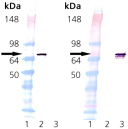
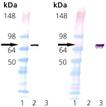
Western Blot analysis: Lane 1: MWM, Lane 2: 100 ng of Bovine Hsc70 (HSP73) Protein, Lane 3: 100 ng of E. coli DnaK Protein. Left: probed with anti-Hsc70 (HSP73) mAb. Right: probed with anti-DnaK mAb.
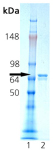
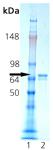
SDS-PAGE analysis: Lane 1: MW marker, Lane 2: 0.5µg of Recombinant Hsc70 (HSP73) Protein.
Please mouse over
Product Literature References
Overexpression of human BAG3P209L in mice causes restrictive cardiomyopathy: K. Kimura, et al.; Nature
12, 3575 (2021),
Abstract;
Full Text
Regulation of Hippo pathway by Hsp70-Bag3 complex: Bag3 modulates YAP phosphorylation and its nuclear translocation: S. Baldan, et al.; J. Cell Sci.
134, jcs.259107 (2021),
Abstract;
Evidence that Hsc70 is associated with CNV particles and plays a role in CNV particle disassembly: S.B. Alam & D. Rochon; J. Virol.
91, e01555 (2017),
Abstract;
Modulation of deregulated chaperone-mediated autophagy by a phosphopeptide: C. Macri, et al.; Autophagy
11, 472 (2015),
Abstract;
Full Text
Selection and identification of ligand peptides targeting a model of castrate-resistant osteogenic prostate cancer and their receptors: J. Mandelin, et al.; PNAS
112, 3776 (2015),
Application(s): Western Blotting,
Abstract;
Full Text
Mutations in the substrate binding site of human heat-shock protein 70 indicate specific interaction with HLA-DR outside the peptide binding groove: K.M. Rohrer, et al.; Immunology
142, 237 (2014),
Abstract;
Full Text
The cyclopentenone prostaglandin 15-deoxy-delta(12,14)- PGJ2 attenuates the development of colon injury caused by dinitrobenzene sulphonic acid in the rat: C. Thiemermann, et al. ; Br. J. Pharmacol.
138, 678 (2003),
Application(s): WB ,
Abstract;
Tissue-specific expression of inducible and constitutive Hsp70 isoforms in the western painted turtle: L.T. Buck, et al. ; J. Exp. Biol.
206, 303 (2003),
Application(s): WB ,
Abstract;
Patterns of variation in levels of hsp70 in natural rocky shore populations from microscales to mesoscales: P.M. Halpin, et al.; Integr. Comp. Biol.
42, 815 (2002),
Application(s): WB ,
Abstract;
Possible association of non-binding of HSP70 to HLA-DRB1 peptide sequences and protection from rheumatoid arthritis: G.E. Dannecker, et al. ; Immunogenetics
54, 67 (2002),
Abstract;
Small glutamine-rich protein/viral protein U-binding protein is a novel cochaperone that affects heat shock protein 70 activity: A.T. Panganiban, et al. ; Cell Stress Chaperones
7, 258 (2002),
Application(s): WB ,
Abstract;
Related Products