Product Details
| Alternative Name: | Heat shock protein 60, 60kDa Chaperonin, Protein Cpn60 GroEL protein, HSP60 |
| |
| MW: | ~60kDa |
| |
| Source: | Produced in E. coli. |
| |
| UniProt ID: | P0A6F5 (strain K12) |
| |
| Formulation: | Liquid. In 50mM TRIS-HCl, pH 7.5, containing 100 mM sodium chloride, 10mM magnesium chloride, 5mM DTT, and 0.05% sodium azide. |
| |
| Purity: | ≥90% (SDS-PAGE; Western blot) |
| |
| Purity Detail: | Purified by multi-step chromatography. |
| |
| Applications: | ELISA, WB
Activity assay, Antigen microarray, in vitro Assay
|
| |
| Application Notes: | ATPase activity assay (positive). Western blot control. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Scientific Background: | In its native form, GroEL exists as a 14-mer consisting of two stacked rings each composed of seven identical subunits of Mw ~57,250. Molecular chaperones, such as GroEL and GroES, are thought to function by interacting with transiently exposed interactive peptide surfaces during its nascent synthesis, folding, transport and oligomerization. The net effect of chaperone participation in these processes is a reduction in inappropriate protein-protein interactions that might produce a nonfunctional structure. The GroEL/GroES complex has a high affinity for peptides during ATP hydrolysis when protein substrates would undergo repeated cycles of assisted folding. The GroEL and GroES genes, constituting the GroE operon, are members of the heat shock regulon ofE. coli. Synthesis of the GroE protein, which accounts for approximately 1% of the cellular protein at 37oC, increases to about 10% of the total protein content upon shifting the growth temperature to 46oC. In vivo and in vitro studies have demonstrated that the GroE system participates in facilitating protein folding/ renaturation, for example: 1) Both GroEL and GroES are required for in vivo assembly of the prokaryotic ribulose bisphosphatase carboxylases (RuBisCo’s) inE. coli, 2) Mutations in the groE locus have been shown to interfere with the assembly of heads of bacteriophages lambda and T 2, 3) Chemically denatured citrate synthase refolds to an active, nonaggregated form in the presence of GroEL, GroES, and Mg-ATP, 4) The sigma-subunit of Streptomyces coelicolor RNA polymerase can be renatured in the presence of GroEL after elution from a SDS-polyacrylamide gel, 5) GroEL and GroES facilitated the in vitro refolding of the monomeric mitochondrial enzyme rhodanese, 6) The aggregation of the non-native states(s) of oat phytochrome can be suppressed by interaction with GroEL and a stable binary complex can form between the two proteins. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
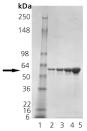
SDS-PAGE analysis: Lane 1: MWM, Lane 2: 0.5μg; Lane 3: 1μg; Lane 4: 2μg; Lane 5: 5μg of Prod. No. ADI-SPP-610 detected by Coomassie Stain.
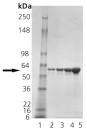
Western Blot analysis: Lane 1: MW marker, Lane 2: 100 ng of Prod. No. ADI-SPP-610 probed with Prod. No. ADI-SPS-870.
Please mouse over
Product Literature References
Bacterial Biofilm Components Induce an Enhanced Inflammatory Response Against Metal Wear Particles: U. Dapunt, et al.; Ther. Clin. Risk Manag.
16, 1203 (2020),
Abstract;
Full Text
Bacterial Heat Shock Protein GroEL (Hsp64) Exerts Immunoregulatory Effects on T Cells by Utilizing Apoptosis: A.M. Nalbant, et al.; PLoS One
11, e0164380 (2016),
Application(s): Preparation of the GroEL antigen,
Abstract;
Full Text
Antigen microarrays identify unique serum autoantibody signatures inclinical and pathologic subtypes of multiple sclerosis: H. Weiner, et al. ; PNAS
105, 18889 (2008),
Application(s): Antigen Microarray ,
Abstract;
Different heat shock protein 60 species share pro-inflammatory activity but not binding sites on macrophages: H. Kolb, et al. ; FEBS Lett.
533, 105 (2003),
Application(s): In Vitro Assay ,
Abstract;
Serological association between Chlamydia pneumoniae infection and age-related macular degeneration: S. Mansour, et al. ; Arch. Ophthalmol.
121, 478 (2003),
Application(s): EIA ,
Abstract;
Serum immunoglobulin g antibodies to chlamydial heat shock protein 60 but not to human and bacterial homologs are associated with coronary artery disease: G.I. Byrne, et al. ; Circulation
106, 1659 (2002),
Application(s): EIA ,
Abstract;
Related Products