Product Details
| Alternative Name: | HSP75, Mitochondrial HSP90, TNF receptor-associated protein 1, MtHSP90 |
| |
| Clone: | 9B6 |
| |
| Host: | Mouse |
| |
| Isotype: | IgG2a |
| |
| Immunogen: | Recombinant human TRAP1. |
| |
| UniProt ID: | Q12931 |
| |
| Source: | Purified from hybridoma tissue culture supernatant. |
| |
| Species reactivity: | Human, Mouse
|
| |
| Applications: | WB
|
| |
| Recommended Dilutions/Conditions: | Western Blot (1:500, ECL)
Suggested dilutions/conditions may not be available for all applications.
Optimal conditions must be determined individually for each application. |
| |
| Application Notes: | Detects a band of ~72kDa by Western blot. |
| |
| Purity Detail: | Protein G affinity purified. |
| |
| Formulation: | Liquid. In PBS, pH 7.2, containing 50% glycerol and 0.09% sodium azide. |
| |
| Handling: | Avoid freeze/thaw cycles. |
| |
| Shipping: | Blue Ice |
| |
| Long Term Storage: | -20°C |
| |
| Scientific Background: | The Hsp90 family of heat shock proteins represents one of the most abundantly expressed and highly conserved families of cellular chaperones whose expression can be upregulated under conditions of cellular stress. It includes cytoplasmic (Hsp90-alpha/beta), ER (Grp94) and mitochondrial (TRAP1) localized members. Hsp90 is structurally composed of an N-terminal ATP binding domain, a medial substrate-binding region, and a C-terminal dimerization motif. Hsp90 dimers function in cooperation with cochaperones to stabilize a multitude of client protein substrates. TRAP1 (aka Hsp75) was originally identified as a chaperone binding partner for retinoblastoma protein. TRAP1 was later found by immunofluorescence to be localized to the mitochondria. TRAP1 is significantly more active as an ATPase due in part to the lack of regulatory elements in the C-terminus, but retains the ability to dimerize. However, it does not form stable complexes with typical cochaperones of Hsp90 like p23 and HOP4. Recently TRAP1 has been identified as a protective element for cell survival, and has been suggested as a novel target for anti-cancer therapeutics. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
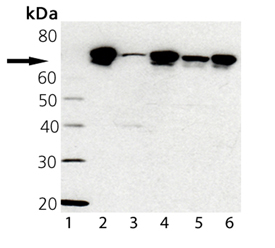
Western blot analysis of TRAP1 probed with TRAP1, mAb (9B6) (Prod. No. ADI-SPA-971): Lane 1: MWM, Lane 2: TRAP1 Recombinant Human Protein (Prod. No. ADI-SPP-848), Lane 3: HL-60 Cell Lysate, Lane 4: Hep G2 Cell Lysate, Lane 5: 3T3 Cell Lysate, Heat Shocked (Prod. No. ADI-LYC-3T101), Lane 6: HeLa Cell Lysate, Heat Shocked (Prod. No. ADI-LYC-HL101)
Please mouse over
Product Literature References
Histone deacetylase activity mediates acquired resistance towards structurally diverse HSP90 inhibitors: R.C. Chai, et al.; Mol. Oncol.
11, 567 (2017),
Abstract;
Full Text
General Literature References
Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer: I. Leav, et al.; Am. J. Pathol.
176, 393 (2010),
Abstract;
TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells: E. Costantino, et al.; Cancer Lett.
279, 39 (2009),
Abstract;
Regulation of heat shock protein 90 ATPase activity by sequences in the carboxyl terminus: B.A. Owen, et al.; J. Biol. Chem.
277, 7086 (2002),
Abstract;
The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties: S.J. Felts, et al.; J. Biol. Chem.
275, 3305 (2000),
Abstract;
The charged region of Hsp90 modulates the function of the N-terminal domain: T. Scheibel, et al.; PNAS U.S.A.
96, 1297 (1999),
Abstract;
Oligomeric forms of the 90-kDa heat shock protein: T. Nemoto & N. Sato; Biochem. J.
330, 989 (1998),
Abstract;
A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock: C.F. Chen, et al.; Mol. Cell. Biol.
1, 4691 (1996),
Abstract;
Related Products