Product Details
| Alternative Name: | HSP86, Heat shock protein 90 |
| |
| Host: | Rabbit |
| |
| Immunogen: | Recombinant human HSP90β. |
| |
| UniProt ID: | P08238 |
| |
| Source: | Purified from rabbit serum. |
| |
| Species reactivity: | Human, Mouse
Bovine, Dog, Guinea pig, Hamster, Porcine, Rabbit, Sheep
|
| |
| Applications: | WB
|
| |
| Recommended Dilutions/Conditions: | Western Blot (1:1,000, colorimetric)
Suggested dilutions/conditions may not be available for all applications.
Optimal conditions must be determined individually for each application. |
| |
| Application Notes: | Detects a band of ~90kDa by Western blot. |
| |
| Purity Detail: | Protein A affinity purified. |
| |
| Formulation: | Liquid. In PBS containing 50% glycerol and 0.09% sodium azide. |
| |
| Handling: | Avoid freeze/thaw cycles. |
| |
| Shipping: | Blue Ice |
| |
| Long Term Storage: | -20°C |
| |
| Scientific Background: | The 90 kDa molecular chaperone family includes 90 kDa heat shock protein Hsp90 and 94 kDa glucose-regulated protein grp94, both major molecular chaperones of the cytosol and the endoplasmic reticulum. Mammalian cells express inducible Hsp90 alpha and constitutive Hsp90 beta isoforms that are encoded by separate genes. The amino acid sequences of human and yeast Hsp90 alpha are 85% and 90% homologous to those of Hsp90 beta, respectively. All known members of the Hsp90 protein family are highly conserved, especially in the N-terminal and C-terminal regions containing independent chaperone sites with different substrate specificity. These ubiquitous and highly conserved proteins account for 1-2% of all cellular protein in most cells. Hsp90 functions as part of the cell's powerful network of chaperones to fight the deleterious consequences of protein unfolding caused by non-physiological conditions. In the absence of stress, however, Hsp90 provides a necessary component of such fundamental cellular processes as hormone signaling and cell cycle control by serving as a chaperone for many key signaling molecules including steroid receptors, cell cycle kinases involved in signal transduction, and p53. As many of these client proteins are known oncogenes, Hsp90 inhibitors such as 17-AAG, a geldanamycin analog, have been of benefit in the treatment of many cancers. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
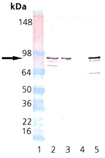
Western blot analysis of HSP90 beta pAb: Lane 1: MW marker, Lane 2: HeLa Cell Lysate, Heat Shocked (Prod. No. ADI-LYC-HL101), Lane 3: HSP90 Native Human Protein (Prod. No. ADI-SPP-770), Lane 4: HSP90 alpha Recombinant Human Protein (Prod. No. ADI-SPP-776), Lane 5: HSP90 beta Recombinant Human Protein (Prod. No. ADI-SPP-777).
Please mouse over
Product Literature References
S-glutathionylation of Hsp90 enhances its degradation and correlates with favorable prognosis of breast cancer: Y.Y. Shih, et al.; Redox Biol.
57, 102501 (2022),
Abstract;
Tau protein aggregates inhibit the protein-folding and vesicular trafficking arms of the cellular proteostasis network: A. Yu, et al.; J. Biol. Chem.
294, 7917 (2019),
Abstract;
Proteomic identification of proteins differentially expressed following overexpression of hTERT (human telomerase reverse transcriptase) in cancer cells: R.K. Jaiswal, et al.; Plos One
12, e0181027 (2017),
Application(s): WB in U2OS and HeLa cells,
Abstract;
Full Text
Related Products










