Product Details
| Alternative Name: | BLVR |
| |
| Host: | Rabbit |
| |
| Immunogen: | Recombinant rat Biliverdin Reductase. |
| |
| UniProt ID: | P46844 |
| |
| GenBank ID: | M81681 |
| |
| Source: | Purified from rabbit serum. |
| |
| Species reactivity: | Human, Mouse, Rat
Hamster, Porcine
|
| |
| Applications: | IHC, WB
|
| |
| Recommended Dilutions/Conditions: | Western Blot (1:1,000, ECL)
Suggested dilutions/conditions may not be available for all applications.
Optimal conditions must be determined individually for each application. |
| |
| Application Notes: | Detects bands of ~33kDa (rat) and ~41-42kDa (human) by Western blot. |
| |
| Purity Detail: | Protein A affinity purified. |
| |
| Formulation: | Liquid. In PBS, pH 7.2, containing 50% glycerol and 0.09% sodium azide. |
| |
| Shipping: | Blue Ice |
| |
| Long Term Storage: | -20°C |
| |
| Scientific Background: | Cleavage of heme b (Fe-protoporphyrin IX) at the a-methene carbon bridge to form the open tetrapyrrole, biliverdin IXa and carbon monoxide (CO) is catalyzed by heme oxygenase (HO) isozymes HO-1 and HO-2 (heme hydrogen-donor: oxygen oxidoreductase; EC 1.14.99.3). In mammalian species, biliverdin reductase (BVR; bilirubin: NAD(P)+ oxidoreductase; EC 1.3.1.24) converts the open tetrapyrrole to bilirubin. This pathway represents the only efficient way of making bilirubin and thereby deterring activation of oxygen by the heme molecule. HO-1 belongs to the heat shock protein family (Hsp32), while HO-2 takes a constitutive form expressed at exceedingly high levels in the brain and testes. The end products of the heme degradation process carry out important physiological activities. CO may act as a messenger in the brain and systemic organs stimulating cGMP-production through interactions with the heme-dependent form of guanylate cyclase. Bile pigments display potent antioxidant activity as well as effective antiviral activity against HIV and herpes virus. BVR is unique among all enzymes characterized to date in having two pH optima (6.8 and 8.7), using a different cofactor at each pH range (NADH at pH 7.0 and NADPH at pH 8.7). The enzyme displays pI and molecular mass microheterogeneity, apparently a result of post translational modifications. In rat, the enzyme also shows a tissue specific developmental pattern. BVR is not inactivated by heat shock, and its preexisting message is not sequestered from translation subsequent to thermal stress. Furthermore, reductase preserves microheterogeneity under thermal stress. BVR expression occurs not only in cells and brain regions that already display HO-1 and HO-2, but also in regions and cell types with potential to induce stress proteins. Rat cDNA for BVR has been isolated and characterized. The deduced protein contains 3 cysteine residues (Cys73, Cys281, and Cys290) involved in cofactor and substrate binding. Human BVR consists of a substantially longer polypeptide than the rat enzyme (41-42 kDa vs. 33 kDa), but also is dual cofactor and dual pH dependent, requires free SH groups for activity, and displays pI and molecular mass microheterogeneity. The human and rat BVR share some antigenic epitopes and show immunochemical cross reactivity. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
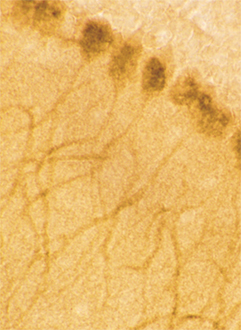
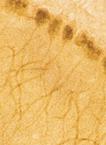
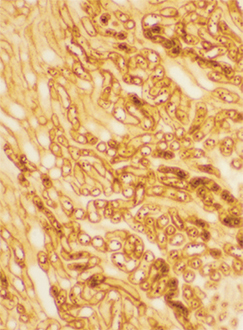
Frozen rat kidney cortex section after ischemic injury, stained using Biliverdin Reductase pAb.
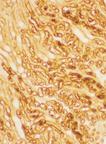
Mouse brain purKinje cells after ischemic injury, stained using Biliverdin Reductase pAb
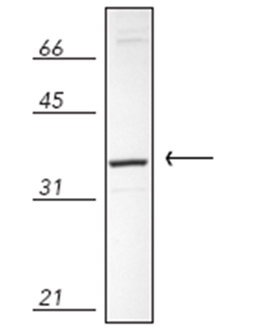
Western blot analysis of rat embryo Rat-2 cell lysate, probed with Biliverdin Reductase pAb
Please mouse over
Product Literature References
Bilirubin gates the TRPM2 channel as a direct agonist to exacerbate ischemic brain damage: H.W. Liu, et al.; Neuron
111, 1609 (2023),
Abstract;
Immunohistochemical localization of the antioxidant enzymes biliverdin reductase and heme oxygenase-2 in human and pig gastric fundus: R.A. Lefebvre, et al. ; Free Radic. Biol. Med.
32, 630 (2002),
Application(s): IHC using porcine, rat & human samples,
Abstract;
Investigation of the potential modulatory effect of biliverdin, carbon monoxide and bilirubin on nitrergic neurotransmission in the pig gastric fundus: R.A. Lefebvre, et al. ; Eur. J. Pharmacol.
457, 177 (2002),
Application(s): IHC using porcine samples,
Abstract;
Spin trap (N-t-butyl-alpha-phenylnitrone)-mediated suprainduction of heme oxygenase-1 in kidney ischemia/reperfusion model: role of the oxygenase in protection against oxidative injury: N. Panahian, et al. ; J. Pharmacol. Exp. Ther.
291, 911 (1999),
Application(s): IHC using rat samples,
Abstract;
Detection of 10 variants of biliverdin reductase in rat liver by two-dimensional gel electrophoresis: T.J. Huang, et al.; J. Biol. Chem.
264, 7844 (1989),
Application(s): WB using rat samples,
Abstract;