- Cost effective - coat your own plates and stretch your research budget
- Quantitative - obtain fully quantitative statistically significant results
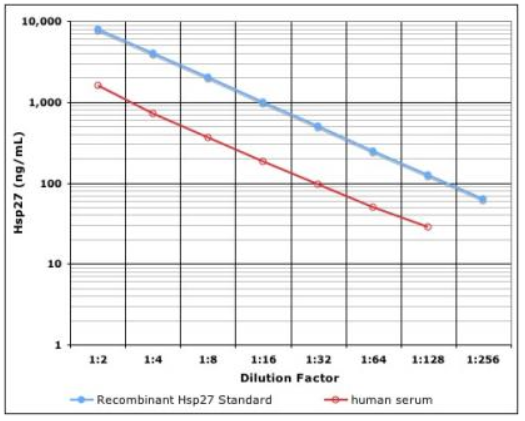
- Ultra-Sensitive - detect as little as 97 pg/mL Hsp27 compared to microgram levels in Western blot
- Reliable - obtain accurate results with thoroughly tested reagents in multiple complex sample matrices
The IMMUNOSET® HSP27 high sensitivity (human), EIA development set contains sufficient reagents for the development of 5 x 96 well EIA plates which provides analysis of ~200 samples in duplicate. Save time, money, and sample by developing your own EIA kits. Simply coat your plates and go! This kit provides superior accuracy with fully quantitative results compared to Western blot analysis and has low reactivity with related molecules.
Please mouse over
Product Details
| Alternative Name: | Heat shock protein 27 |
| |
| Sensitivity: | 97 pg/ml (range 0.1 - 3.2 ng/ml) |
| |
| Assay Time: | Plate Coating - Overnight + 1 hour; Assay - 2 hours 45 minutes |
| |
| Applications: | ELISA, Colorimetric detection
|
| |
| Application Notes: | For the quantitative determination of human HSP27 in cell lysates, plasma, and serum. |
| |
| Wavelength: | 450 nm |
| |
| Species reactivity: | Human
|
| |
| Crossreactivity: | Specific for HSP27. Does not cross-react with HSP25, Cpn10, HO-1, HSP40, or αB-crystallin. |
| |
| Contents: | Capture antibody, Standard, Detection antibody, SA-HRP |
| |
| Shipping: | Blue Ice Not Frozen |
| |
| Long Term Storage: | +4°C |
| |
| Scientific Background: | Hsp27 is one of the most common members of the highly conserved and ubiquitously expressed family of small heat shock proteins (sHsp), which also includes αB-crystallin. It is characterized by a conserved C-terminal α-crystallin domain consisting of two anti-parallel β-sheets that promote oligomer formation required for its primary chaperone function as inhibitor of irreversible protein aggregation. Hsp27 oligomerization is modulated by post-translational phosphorylation of Hsp27 at three serine residues, Ser15, Ser78, and Ser82, by a variety of protein kinases including MAPKAPK-3, PKAc-α, p70 S6K, PKD I, and PKC-δ. Hsp27 has been shown to inhibit actin polymerization by binding of unphosphorylated Hsp27 monomers to actin intermediate filaments. Anti-apoptotic functions of Hsp27 have also been identified through interactions with DAXX7, activation of Akt, and inhibition of apoptosome formation. Evidence suggests altered expression of Hsp27 is implicated in the pathogenesis of breast, ovarian, and prostate cancer. |
| |
| UniProt ID: | P04792 |
| |
| GenBank ID: | L39370 |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
Impact of hyperthermic intraperitoneal chemotherapy on Hsp27 protein expression in serum of patients with peritoneal carcinomatosis: V. Kepenekian, et al.; Cell Stress Chaperones
18, 623 (2013),
Abstract;