Replaces Prod. #: ALX-270-368
Inhibitor of PPARα activation. Inhibits 5-lipoxygenase-activating protein (FLAP) and leukotriene biosynthesis. Binds to FLAP with high affinity and prevents the activation of 5-lipoxygenase. IC50’s for leukotriene biosynthesis: 2.5 nM in intact leukocytes; 1.1 µM in whole blood. Induces apoptosis through the mitochondrial pathway.
Product Details
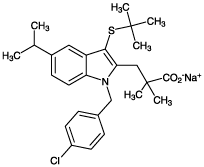
| Alternative Name: | L-663,536, 3-[1-(p-Chlorobenzyl)-5-(isopropyl)-3-t-butylthioindol-2-yl]-2,2-dimethylpropanoic acid . Na |
| |
| Formula: | C27H33ClNO2S·Na |
| |
| MW: | 494.0 |
| |
| CAS: | 118414-82-7 |
| |
| Purity: | ≥98% (HPLC) |
| |
| Appearance: | White solid. |
| |
| Solubility: | Soluble in 100% ethanol (25mg/ml) or DMSO (25mg/ml). |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | Ambient |
| |
| Use/Stability: | Stable for at least 1 year after receipt when stored, as supplied, at room temperature. Prepare fresh solutions daily. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
Mitochondria are direct targets of the lipoxygenase inhibitor MK886. A strategy for cell killing by combined treatment with MK886 and cyclooxygenase inhibitors: A. Gugliucci, et al.; J. Biol. Chem.
277, 31789 (2002),
Abstract;
Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886: J.P. Kehrer, et al.; Biochem. J.
356, 899 (2001),
Abstract;
FLAP: a novel drug target for inhibiting the synthesis of leukotrienes: A.W. Ford-Hutchinson; Trends Pharmacol. Sci.
12, 68 (1991),
Abstract;
Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis: R.A. Dixon, et al.; Nature
343, 282 (1990),
Abstract;
L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor: J. Gillard, et al.; Can. J. Physiol. Pharmacol.
67, 456 (1989),
Abstract;