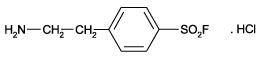
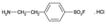
Specific irreversible inhibitor of serine proteases including trypsin, chymotrypsin, plasmin, plasma kallikrein, and thrombin. Stable, non-toxic alternative to PMSF (Prod. No. ALX-270-184). Inhibitor of NAD(P)H oxidase. Activator of MAP kinases JNK and p38.
Product Details
| Alternative Name: | 4-(2-Aminoethyl)benzenesulfonylfluoride . HCl, Pefabloc®SC |
| |
| Formula: | C8H10FNO2S . HCl |
| |
| MW: | 239.7 |
| |
| CAS: | 30827-99-7 |
| |
| Purity: | ≥98% (HPLC) |
| |
| Appearance: | White to off-white powder. |
| |
| Solubility: | Soluble in water or 100% ethanol. |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | +4°C |
| |
| Handling: | Protect from moisture and light. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
UBQLN2 undergoes a reversible temperature-induced conformational switch that regulates binding with HSPA1B: ALS/FTD mutations cripple the switch but do not destroy HSPA1B binding: T.H. Phung, et al.; Biochim. Biophys. Acta Gen. Subj.
1867, 130284 (2023),
Abstract;
Bifunctional hetero di-disc for broad-spectrum influenza neutralization: S. Moon, et al.; Nanomedicine
44, 102587 (2022),
Abstract;
AMP-activated protein kinase Is activated by non-steroidal anti-inflammatory drugs: T.S. King, et al.; Eur. J. Pharmacol.
762, 299 (2015),
Application(s): Western Blot,
Abstract;
TANK-binding kinase 1 (TBK1) modulates inflammatory hyperalgesia by regulating MAP kinases and NF-κB dependent genes: C. V. Möser, et al.; J. Neuroinflammation
12, 100 (2015),
Application(s): Western Blot,
Abstract;
Full Text
Inhibitory effect of 4-(2-aminoethyl)-benzenesulfonyl fluoride, a serine protease inhibitor, on PI3K inhibitor-induced CHOP expression: T. Hosoi, et al.; Eur. J. Pharmacol.
554, 8 (2007),
Abstract;
Heme oxygenase-1 gene activation by the NAD(P)H oxidase inhibitor 4-(2-aminoethyl) benzenesulfonyl fluoride via a protein kinase B, p38-dependent signaling pathway in monocytes: N. Wijayanti, et al.; J. Biol. Chem.
280, 21820 (2005),
Abstract;
Full Text
Evaluation of two inhibitors of invasion: LY311727 [3-(3-acetamide-1-benzyl-2-ethyl-indolyl-5-oxy)propane phosphonic acid] and AEBSF [4-(2-aminoethyl)-benzenesulphonyl fluoride] in acute murine toxoplasmosis: R. Buitrago-Rey, et al.; J. Antimicrob. Chemother.
49, 871 (2002),
Abstract;
Full Text
4-(2-Aminoethyl)benzenesulfonyl fluoride attenuates tumor-necrosis-factor-alpha-induced blood-brain barrier opening: P. Megyeri, et al.; Eur. J. Pharmacol.
374, 207 (1999),
Abstract;
Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds: V. Diatchuk, et al.; J. Biol. Chem.
272, 13292 (1997),
Abstract;
Full Text
Inhibition of amyloid beta-protein production in neural cells by the serine protease inhibitor AEBSF: M. Citron, et al.; Neuron
17, 171 (1996),
Abstract;
Lysis of leukemic cells by human macrophages: inhibition by 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF), a serine protease inhibitor: Y. Nakabo & M.J. Pabst; J. Leukoc. Biol.
60, 328 (1996),
Abstract;
Full Text
Serine protease inhibitors block priming of monocytes for enhanced release of superoxide: P. Megyeri, et al.; Immunology
86, 629 (1995),
Abstract;
Full Text
Irreversible enzyme inhibitors. 180. Irreversible inhibitors of the C'la component of complement derived from m-(phenoxypropoxy)benzamidine and phenoxyacetamide: B.R. Baker & H.J. Cory; J. Med. Chem.
14, 119 (1971),
Abstract;
Related Products