Vaccine
Creating Efficiency & Maintaing Quality to
Accelerate Your Vaccine Manufacturing

Vaccine-driven immunity is a multifaceted puzzle. The focus on designing antigens stems from our evolving comprehension of how they trigger diverse immune responses. Moreover, the rapid rise of messenger RNA (mRNA) vaccines, built on groundbreaking mRNA technology, has ushered in new possibilities for treating conditions like cancer and infectious diseases.
Vaccines encompass diverse modalities, incorporating live attenuated or inactivated organisms, as well as formulations derived from partially purified components. The fore�front of vaccine innovation now involves the development of RNA and DNA vaccines, repre�senting the latest strides in harnessing genetic material to induce targeted immune responses.
Whether your vaccine development journey involves bacterial, viral, or nucleic acid molecules, we provide the tools to improve yields, lower costs, ensure quality, and cut down your manufacturing process.
“
High-quality vaccine research and manufacturing products that consistently yield reproducible results, ensuring efficiency in time, effort and cost reduction in every lot.
“
Creating Efficiency and Maintaining Quality to Accelerate Vaccine Manufacturing
Selection of antigens for vaccine development requires identifying and preparing specific molecules that mimic the target pathogen to trigger a protective immune response.
Separating antigens from impurities is a pivotal step, safeguarding the purity and safety of the ultimate vaccine formulation.
Vaccine formulation directly impacts the product's safety, efficacy, and stability to ensure that it can effectively protect against the targeted disease.
Monitoring product integrity, especially protein aggregation during formulation, facilitates the refinement of manufacturing processes to maintain strict regulatory standards protective immunity.
Monitoring the immune response to the introduction of antigen allows to assess the effectiveness of vaccines and tailor strategies for enhancing protective immunity.
Antigen Selection

Optimized Expression Systems Deliver Superior Antigenicity
Generating and validating antigens for vaccine development requires selecting and preparing specific molecules that mimic the target pathogen to trigger a protective immune response.
Enzo offers various native-state bacterial and viral antigens for vaccine research. These are native or recombinant proteins produced in the latest expression systems to ensure high-level expression, maintenance of their native-folded state, and retention of all relevant post-translational modifications (PTM).
Native Bacterial and Viral Antigens
- Higher specificity and sensitivity
- Reproducible scale-up and lot-to-lot consistency
- Lyophilized format for extended shelf-life
Pertussis Toxin

A model is built using data of molecular structure from CPs Undetected HCPs Protein Data Bank (PDB 1PRT)
Separation of Antigen from Impurities

Contamination Monitoring
The challenge in vaccine manufacturing lies in effectively monitoring and managing contamination. Separating antigens from impurities is a pivotal step, safeguarding the purity and safety of the ultimate vaccine formulation.
Enzo offers Host Cell Protein (HCP) ELISA kits and reagents for contamination monitoring that are ideal for impurity analysis.
Monitor CHO HCP Residuals with Confidence

CHO Host Cell ELISA Protein Kit was compared to the leading competitor's kit. Results indicate that Enzo's ELISA kit has 25% more coverage than the leading competitor's kit
Formulation

Immunostimulants to Maximize Response
A robust and targeted immune response is essential for the vaccine to provide protection against the targeted disease.
We offer a comprehensive portfolio of products that enhance the magnitude and durability of the vaccine immune response, including MEGACD40L® Protein. CD40 ligand (CD40L) plays a central role in co-stimulation and regulation of the immune response.
MEGACD40L® Protein is a highly active and pure CD40L recombinant protein that can enhance an immune response without additional enhancers.
Improved Stability and Enhanced Activity Compared to CD40L and CD40L with enhancers

B cell lymphocyte activation by various CD40L constructs. PBMCs were treated for 48 hours in media containing serially diluted CD40L, CD40L + 2 μg/mL Enhancer, or MEGACD40L® Protein. Cells were dual-stained with α-human CD19–PE and α-human CD86–APC and analyzed by flow cytometry. Data are presented as the percent of CD19+ B cells that co-stain as CD86+.
Monitor Product Integrity

Maintain Product Integrity
Aggregation poses a substantial challenge in vaccine manufacturing. It can impact product quality, safety, and efficacy. Ensuring structural integrity, preventing safety concerns, and optimizing production processes are critical to meeting regulatory standards and ensuring vaccine stability and efficacy.
Product stability can be assessed by monitoring protein aggregation with PROTEOSTAT® Protein Aggregation Assay, allowing the optimization, formulation, and manufacturing processes.
PROTEOSTAT® Dye Yields Much Brighter Signal

Signal intensity of PROTEOSTAT® aggregate sensing dye compared other dyes
Monitor Immune Response

Detect Analytes Accurately with High-Sensitiviy ELISA Kits
Assessing the vaccine's effectiveness by monitoring the immune response when the antigen is introduced is crucial to tailor strategies to enhance protective immunity.
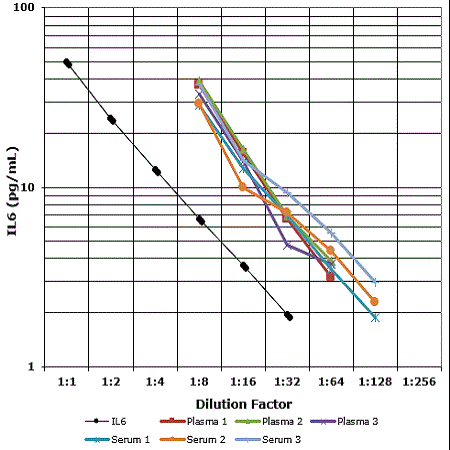
Measuring IL-6 levels in response to vaccination can provide insights into the vaccine's immunostimulatory effects. Enzo offers one of the most sensitive IL-6 ELISA Kits on the market.
- High sensitivity (0.057 pg/mL) colorimetric immunoassay kit available able to detect very low concentrations of IL-6
- Highly specific, showing minimal crossreactivity to IL-1, IL-1αβ, IL-2, IL-3, IL-4, IL-7, IL-8, TNFα, and TNFβ
- Validated with several sample types: plasma, serum, culture supernatants, and urine
Related Products
IFN-γ (human), ELISA kit
IL-8 (human), ELISA kit
IL-1β (human), ELISA kit
TNF-α (human), ELISA kit
IL-10 (human), ELISA kit
cAMP complete ELISA kit
PGE2 high sensitivity ELISA kit
Antibodies
Antibodies are indispensable tools in vaccine research, from initial antigen discovery to vaccine design, efficacy testing, manufacturing, and quality control. They are fundamental in understanding and harnessing the immune response to develop safe and effective vaccines.
LAG-3 is used as a marker in immune monitoring and assessment of vaccine-induced immune responses. Measuring LAG-3 expression on T cells provides insights into the immune system's status and its potential to respond to vaccination.
- A broad range of antibodies for use in all areas of vaccine research
- Validated for use in key applications such as flow cytometry, immunohistochemistry, immunoprecipitation, and Western blotting
- Backed by our Worry-free Antibody Trial Program
Enabling Vaccine Research & Manufacturing

Leverage over 45 years of innovation and technical expertise supporting drug development, discovery, research, and diagnostics through our core technology platforms. With over 150,000 citations, GMP and ISO certifications, our highly-sensitive, quality products consistently deliver trusted results. We maintain product integrity and reliability with our in-house U.S. manufacturing facility. We deploy our bench of expert PhD scientists to provide technical, tailored, support for our customers and their projects.
Rely on our extensive experience in innovation, technology development, and manufacturing to support your vaccine research and development. Our comprehensive Life Sciences Contract Services support the development of customizable, unique, and efficient solutions for all your research needs.
“
Our Technological Depth Enabling Efficiency for our Customers. “
Do You have a Project? Ask our Expert today.
Please fill the form below
We will contact you within 1 business day