
Predictive Toxicology
Innovative Solutions for Early Safety Assessment
Predictive toxicology is a pivotal aspect of modern drug development, utilizing advanced scientific techniques to foresee potential adverse effects of drug candidates. By leveraging in vitro, in silico, and in vivo approaches, predictive toxicology aims to provide early insights into the safety profile of drug candidates, enabling efficient decision-making in drug development. This proactive approach not only mitigates risks but also reduces the likelihood of late-stage failures, saving time and resources.
Enzo’s broad range of scientific expertise and industry-proven manufacturing capabilities provides a comprehensive set of solutions for predictive toxicology when determining genotoxicity, membrane integrity, autophagy, and oxidative stress. Our products are reliable, cost-effective, and compatible with high-throughput capabilities.
With over 45 years of experience and manufacturing excellence, Enzo’s overarching goal is to provide innovative tools that enhance the efficiency, cost-effectiveness, and overall success of drug development endeavors
Enzo's products and services can help to streamline operations, making drug development more accessible and efficient.
Innovative Solutions for Early Safety Assessment
Cell viability testing is crucial for evaluating the impact of compounds on living cells, providing essential insights for safer and more effective drug development.
Understanding cellular dysfunction is crucial in assessing drug candidate safety. Early identification of compounds inducing dysfunction allows for timely adjustments in drug development.
The presence of aggresomes may indicate cellular stress and dysfunction, allowing for the early identification of potential toxic effects induced by drugs or other substances.
Identifying genotoxic drug compounds during early preclinical studies allows for further lead refinement and lower drug attrition.
Focused investigation helps identify and mitigate risks associated with drug-induced harm to specific organs, contributing to the development of safer and more targeted pharmacological interventions.
A comprehensive understanding of drug-induced endocrine and metabolism abnormalities is essential for predicting adverse reactions, guiding drug design, and optimizing treatment strategies.
Cell Viability

Detect Adverse Effects Early On
Evaluating the health of cells through viability testing is fundamental. This essential process provides valuable insights into how compounds may affect the well-being and functionality of living cells, contributing significantly to the quest for a safer and a more effective drug development.
Utilizing Enzo’s advanced in vitro assays, potential toxicity of drug candidates can be assessed early on.
Multiplex Healthy, Early Apoptotic, Late Apoptotic, and Necrotic Cells
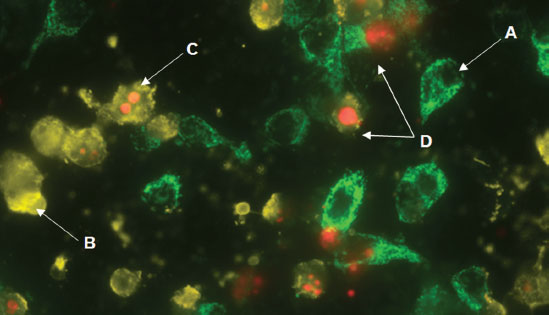
GFP-CERTIFIED® Apoptosis/Necrosis Detection Kit (ENZ-51002)
detects four distinct cell states. Mitochondrial GFP-expressing HeLa cells were treated with 2μM Staurosporine for 4 hours. The apoptosis detection reagent (gold) and necrosis detection reagent (red) specifically detect cell states with clear spectral separation from mitochondria-associated GFP signal. Healthy cells (A), cells undergoing apoptosis (B), cells undergoing late-stage apoptosis (C), and necrotic cells (D).
Cellular Dysfunction

Understand Cellular Toxicity Mechanisms
Understanding how drug candidates may impact critical cellular processes, aids in the prediction of the compound's overall effect on the cell. Compounds that induce significant dysfunction may pose risks to overall organism health, and early identification allows for appropriate adjustments in drug development.
Enzo’s CELLESTIAL® Assays for drug safety & in vitro toxicology provide a comprehensive set of solutions for monitoring cellular processes, aiding in tailoring drug development strategies to enhance therapeutic effectiveness.
MITO-ID® dye is 10X more sensitive than JC-1
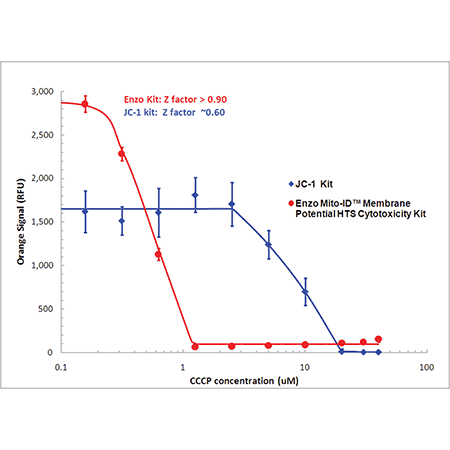
Detect mitochondrial perturbations with 10 times more sensitivity than JC-1. Mitochondrial membrane potential (MMP) was evaluated in HeLa cells treated with CCCP using MITO-ID® dye (red) or JC-1 (blue). Using a conventional fluorescence microplate reader, MMP was shown to decrease with increasing CCCP concentration as indicated by a decrease in orange fluorescence. Improved aqueous solubility of the dye and no-wash protocol minimizes variability, leading to a higher Z-factor (> 0.9) than that obtained with JC-1.
Aggresomal Response

Assess Cellular Stress Response
The presence of aggresomes, or inclusion bodies of misfolded and/or aggregated proteins, is often linked to cellular toxicity. Aggresomes may indicate cellular stress and dysfunction, allowing for the early identification of potential toxic effects induced by drugs or other substances.
PROTEOSTAT® Aggresome Detection Kit
Detect and quantify aggresomes in cells using PROTEOSTAT® Aggresome Detection Kit, a robust and highly sensitive assay for toxicity assessment during drug discovery and development.
- Reliable and simple assay doesn’t require non-physiological protein mutations or genetically engineered cell lines
- Validated with small molecule modulators demonstrating suitability for screening compounds of potential therapeutic value
- Useful for the study of toxicology, neurodegenerative diseases, liver disease, and much more
Related Products
Proteasome ELISA Kit
p62 ELISA Kit
p62 (human) pAb
Proteosome Activator 11S Subunit Ab Sampler Pack
Proteosome Purification Kit
Detection of Aggresomes in HeLa Cells
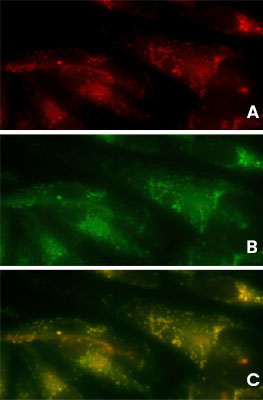
Detection of aggresomes in HeLa cells, treated with proteosome inhibitor MG-132 for 12 hours with (A) PROTEOSTAT® Aggresome Detection Kit (ENZ-51035) (red), (B) aggresome marker p62 antibody conjugated with fluorescein (green).
(C) Composite image shows co-localization of aggresomes and p62 (yellow).
Genotoxicity

Identifying genotoxic drug compounds during early preclinical studies allows for further lead refinement and lower drug attrition. Mutagenesis, carcinogenesis, and adverse genetic effects, especially those that affect the germline, are routinely tested. Common assays for mutagenicity, both in vitro and in vivo, identify mutagenic hazards and mitigate risk for regulatory approval.
Sensitive measurement of DNA Damage
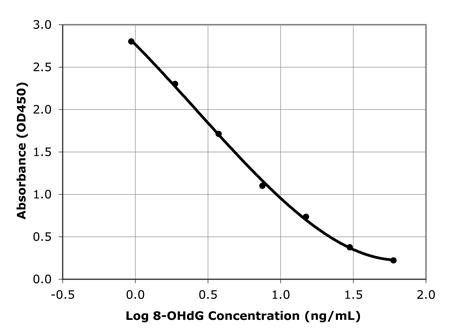
Standard curve of DNA damage ELISA kit detecting as low as 1 ng/ml
of 8-OHdG
Organ-Specific Toxicity

Understanding the potential toxicities directed at specific organs is paramount for ensuring the safety and efficacy of a drug candidate. This focused investigation allows to identify and mitigate risks associated with drug-induced harm to particular organs, contributing to the development of safer and more targeted pharmacological interventions.
Tamoxifen
Tamoxifen, a compound included in Enzo’s SCREEN-WELL® Hepatotoxicity Library, is a selective estrogen receptor modulator (SERM), is commonly used in the treatment of hormone receptor-positive breast cancer. Considered generally safe and effective, it can occasionally cause hepatotoxicity. Tamoxifen and its metabolites can undergo bioactivation by cytochrome P450 enzymes, leading to the formation of reactive intermediates that can damage liver cells.
Metabolic Abnormalities

Drug-induced endocrine and metabolism disorders include electrolyte and calcium abnormalities, carbohydrate metabolism, and thyroid and gonadal disorders. A critical understanding of endocrine effects can better predict drug-induced adverse effects for a better choice of drug design and treatment rationale.
Accurately Detect Cortisol with Exceptional Consistency Across Multiple Production Lots
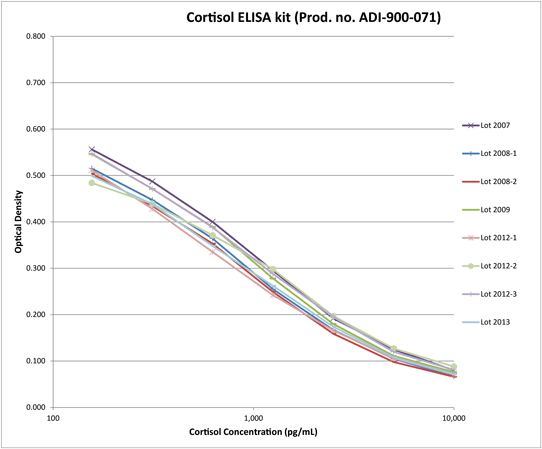
Lot-to-lot consistency graph demonstrates the robust and reproducible nature of the Cortisol ELISA kit showing standard curves from 8 lots manufactured over 7 years.
Enabling Your Safety Assessment

Reduce the risk of your drug discovery pipeline with our cell-based assays, activity kits, and reagents for mechanism and pathway analysis and biomarker detection. Enzo’s broad range of scientific expertise and industry-proven manufacturing capabilities provides a comprehensive set of solutions for the toxicology market when determining cell viability, cellular dysfunction, genotoxicity, organ-specific toxicity and metabolic abnormalities
Enzo's offerings and solutions are designed to enhance operational efficiency, simplifying processes and increasing accessibility in the field of drug development.
“
Facilitating Predictive Toxicology to Ensure Drug Safety “
Do You have a Project? Ask our Expert today.
Please fill the form below
We will contact you within 1 business day
















