-
Sensitive quantitation of HCV viral load
-
Low-cost alternative to other methods of viral load detection
-
Compatible with most open qPCR platforms
-
Complete set of controls including: quantitation controls, an internal sample extraction control, and a negative control
-
Smaller sample input allows remaining extracted samples to be used in other tests
The AMPIPROBE® HCV assay kit is a quantitative reverse transcription polymerase chain reaction (RT-qPCR) assay for the quantitative detection of human hepatitis C virus (HCV) RNA in plasma or serum. The proprietary primer mix included in the kit is specific for HCV genotypes 1 through 6. The kit includes titered high, medium, and low HCV controls which enable virus quantitation in IU/mL. In addition, the kit contains an extraction control to ensure proper sample nucleic acid extraction.
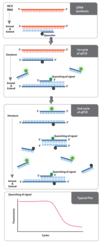
Enzo's AMPIPROBE® technology incorporates probe detection technology in primer design. It employs a combination of fluorescent reporter-labeled primers and quencher-labeled primers to amplify DNA, akin to traditional PCR. When free in solution, fluorescent primers generate a signal. However, as the primers are incorporated into amplified DNA, the quencher and the fluorophore are brought within close proximity and exhibit Forster resonance energy transfer (FRET). This causes a logarithmic decay of signal with respect to the number of amplification cycles of DNA. Once the signal decays to a defined threshold, a value is generated with respect to the corresponding cycle. The threshold cycle is indicative of the amount of target RNA or DNA in the sample.
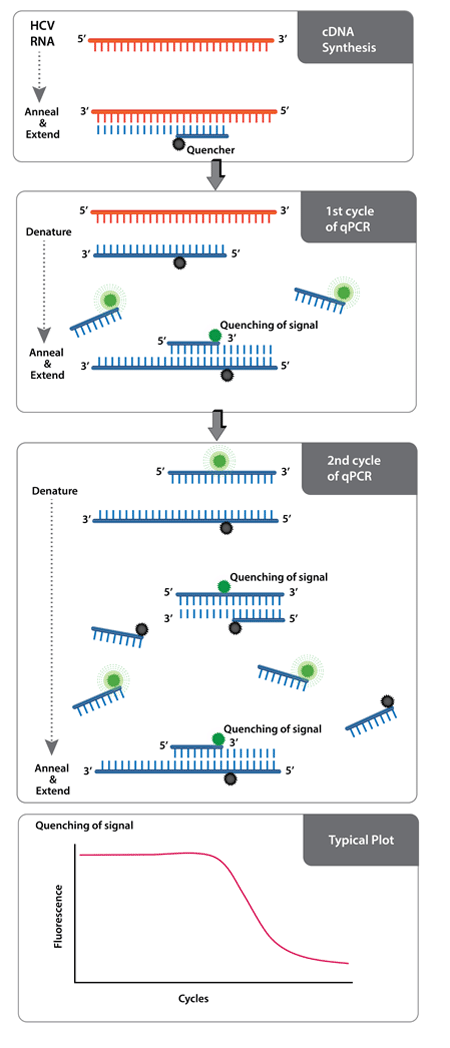
AMPIPROBE® HCV assay kit technology
Please mouse over
Product Details
| Sensitivity: | In a validation study approved by the New York State Department of Health, the AMPIPROBE® HCV assay was determined to have the following sensitivity:
LOD serum - 5.46 IU/mL via 95% Probit analysis
LOD plasma - 7.91 IU/mL via 95% Probit analysis
LOQ serum - 10 IU/mL via 95% hit rate analysis
LOQ plasma - 10 IU/mL via 95% hit rate analysis
In the same validation study, the linear range of both serum and plasma was determined to be 5 to 25,000,000 IU/mL. |
| |
| Applications: | qPCR
|
| |
| Application Notes: | The AMPIPROBE® HCV assay is compatible with any properly calibrated qPCR thermal cycler capable of exciting and reading emissions from FAM/Green and CY5/Red. It has been validated for use on the following instruments: QIAGEN Rotor-Gene Q and Applied Biosystems® 7500. |
| |
| Species reactivity: | Hepatitis C virus
|
| |
| Specificity: | The AMPIPROBE® HCV assay is capable of quantifying viral RNA from HCV genotypes 1-6.
In a validation study approved by the NYS DOH, for genotype inclusivity, the AMPIPROBE® HCV assay detected genotypes 1a, 1b, 2a, 3a, 4acd, 5a, and 6 at concentrations of 15 IU/mL or greater with a hit rate of ≥95%. |
| |
| Use/Stability: | All components are stable at -80°C until the kit's expiration date. |
| |
| Shipping: | Dry Ice |
| |
| Short Term Storage: | -80°C |
| |
| Long Term Storage: | -80°C |
| |
| Contents: | AMPIPROBE® HCV Primer Mix
Negative Control
HCV High Control
HCV Medium Control
HCV Low Control
Internal Control
Card Insert with values of HCV High, Medium, and Low Controls |
| |
| Scientific Background: | HCV is a single-stranded, positive sense RNA virus with a genome of approximately 9,500 nucleotides coding for 3,000 amino acids. HCV exists as six closely related, yet distinct genotypes.
HCV infects about 4 million individuals in the US and about 170 million individuals worldwide. Acute HCV infection will most often progress to chronic infection. If left untreated, the disease may progress to liver fibrosis, cirrhosis, decompensated liver disease, and death. In addition, 25% of all cirrhotic patients will also develop hepatocellular carcinoma.
Quantifying HCV RNA is a well-established method for measuring baseline viral loads and response to treatment. HCV RNA can be detected in plasma or serum by extraction and amplification of nucleic acid. The AMPIPROBE® HCV assay kit uses a novel probe system to accurately quantify HCV RNA. |
| |
| Technical Info/Product Notes: | Application Note
A Novel, High Sensitivity, Quantitative Hepatitis C Virus Assay
|
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Related Products