- A simple, sensitive, homogenous fluorescent assay
- Validated for use with microplate or flow cytometry platform
- Extensively benchmarked with IgG
- Optimize buffers and excipients for protein formulation
- Performs with a wide pH and ionic strength range
- Use with PROTEOSTAT® Protein Aggregation Standards for accurate quantification of aggregated protein in solution.
PROTEOSTAT
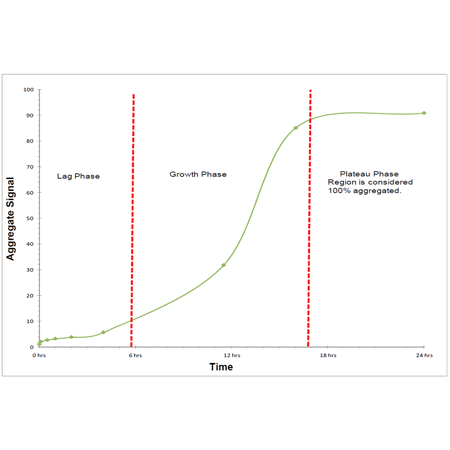
® Protein aggregation assay provides a simple, homogenous assay format for monitoring peptide and protein aggregation in solution. This is useful for defining optimal storage formulations for proteins, for screening of compounds that promote or inhibit protein aggregation and potentially for the sensitive measurement of molecular chaperone activity. The assay can be employed to streamline protein processing and formulation optimization procedures. Relative to conventional protein aggregation detection dyes, such as Thioflavin T, the Enzo PROTEOSTAT
® detection reagent can detect aggregates from a broader range of proteins, yields a much brighter signal, provides at least 2 orders of magnitude linear dynamic range and offers superior performance across a broad range of pH values (4~10) and buffer compositions. Sensitivity for this assay is in the sub-micromolar range and as little as 1-5% protein aggregate is detectable in a concentrated protein solution. The assay is capable of providing quantitative analysis of protein aggregation in a robust and high-throughput fashion (Z’ factor score >0.5). Lyophilized native and aggregated protein are provided as negative and positive controls for monitoring changes in protein aggregation status.
PROTEOSTAT
® Protein Aggregation Standards kit (Prod. No.
ENZ-51039) is the
only commercially available protein aggregation standards assay with stabilized, high-quality reference samples for generating trace protein aggregate levels in concentrated monomeric IgG.
Easy to use -
simply add water!
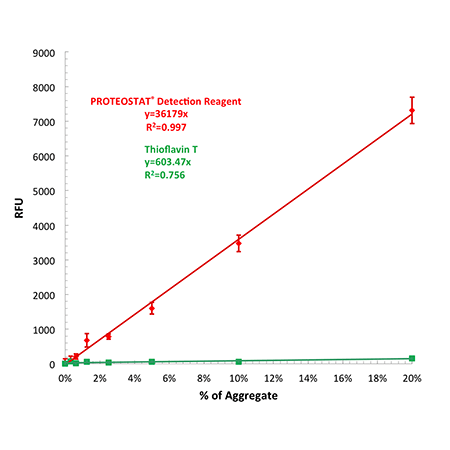
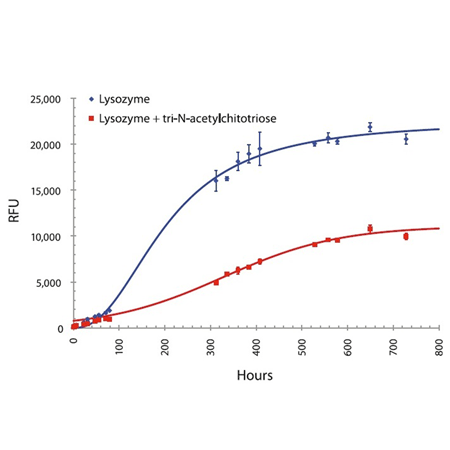
Effective linear dynamic range for antibody aggregate detection using PROTEOSTAT® Detection Reagent compared with Thioflavin T. Relative fluorescence unit values (RFUs) may differ depending upon the microplate reader employed for the analysis.
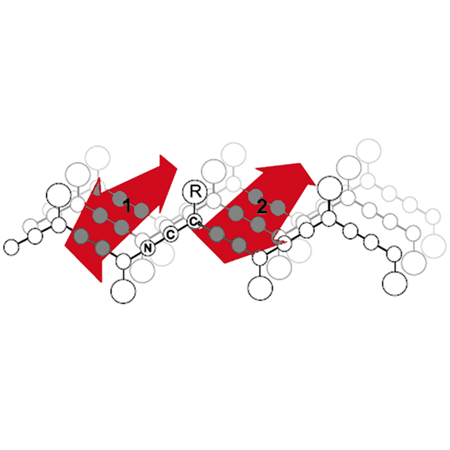
Dye is immobilized when bound to the aggregate and begins to fluoresce.
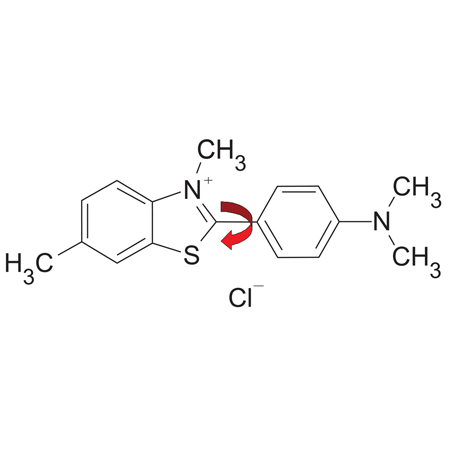
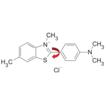
Thioflavin T: early prototype dye in the design of PROTEOSTAT® assay which also rotates around a single bond (red arrow) in the absence of protein aggregates.
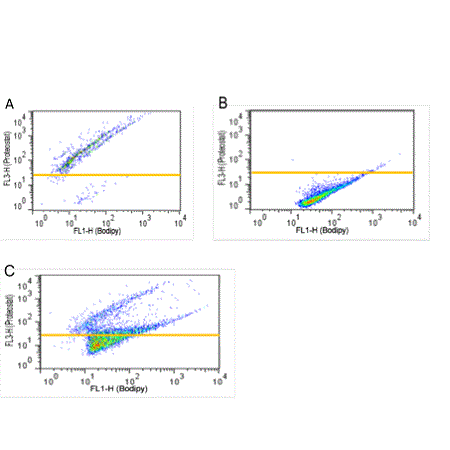
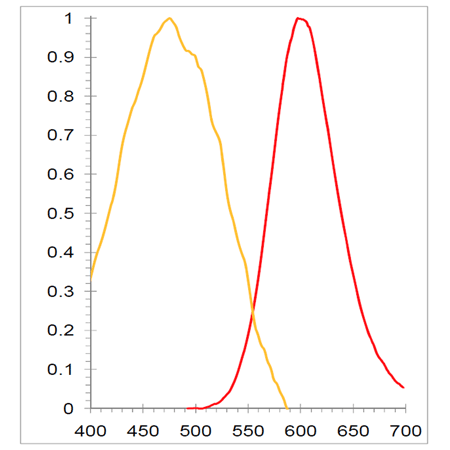
Figure 2. Flow Cytometry Application: PROTEOSTAT® Dye Mixed with Bodipy Dye (Pyrromethene 546) Differentiates Oil Droplets From True Protein Aggregates. Bodipy vs PROTEOSTAT® fluorescence of IgG aggregates (A), Silicon oil droplets (B) and a mixture of Silicon oil droplets and IgG aggregates (C).
Please mouse over
Product Details
Product Literature References
Amyloid-like RIP1/RIP3 RHIM Fragments’ Characterization and Application as a Drug Depot: M. Ismail, et al.; Molecules
28, 1480 (2023),
Abstract;
DNMT2/TRDMT1 gene knockout compromises doxorubicin-induced unfolded protein response and sensitizes cancer cells to ER stress-induced apoptosis: J.A. Grochala, et al.; Apoptosis
28, 166 (2023),
Abstract;
EVA1A regulates hematopoietic stem cell regeneration via ER-mitochondria mediated apoptosis: B. Liu, et al.; Cell Death Dis.
14, 71 (2023),
Abstract;
Inverse design of viral infectivity-enhancing peptide fibrils from continuous protein-vector embeddings: K. Kaygisiz, et al.; Biomater. Sci.
11, 5251 (2023),
Abstract;
The protective effect of erinacine A-enriched Hericium erinaceus mycelium ethanol extract on oxidative Stress–Induced neurotoxicity in cell and Drosophila models of spinocerebellar ataxia type 3: Y.L. Wu, et al.; Free Radic. Biol. Med.
195, 1 (2023),
Abstract;
Ubiquitin-like protein MNSFβ regulates glycolysis and promotes cell proliferation with HSC70 assistance: M. Nakamura, et al.; Biochem. Biophys. Rep.
33, 101414 (2023),
Abstract;
4-phenylbutyric acid - Identity crisis; can it act as a translation inhibitor?: D. Stein, et al.; Aging Cell
21, e13738 (2022),
Abstract;
Exposure of α-Synuclein Aggregates to Organotypic Slice Cultures Recapitulates Key Molecular Features of Parkinson's Disease: S. Moudio, et al.; Front. Neurol.
13, 826102 (2022),
Abstract;
GrpEL1 regulates mitochondrial unfolded protein response after experimental subarachnoid hemorrhage in vivo and in vitro: C. Ma, et al.; Brain Res. Bull.
181, 97 (2022),
Abstract;
Identification of an endoplasmic reticulum proteostasis modulator that enhances insulin production in pancreatic β cells: M. Miyake, et al.; Cell Chem. Biol.
1016, S2451 (2022),
Abstract;
Photoinduced Amyloid Fibril Degradation for Controlled Cell Patterning: K. Kaygisiz, et al.; Macromol. Biosci.
0.1002, e2200294 (2022),
Abstract;
Protocol for isolation and proteostatic analysis of sub-populations of spermatogenic cells in mouse: Q. Zou, et al.; STAR Protoc.
3, 101398 (2022),
Abstract;
Quantitative super-resolution imaging of pathological aggregates reveals distinct toxicity profiles in different synucleinopathies: M.J. Morten, et al.; PNAS
119, e2205591119 (2022),
Abstract;
Surface cysteine to serine substitutions in IL-18 reduce aggregation and enhance activity: J. Saetang, et al.; Peer J.
10, e13626 (2022),
Abstract;
The protein aggregation inhibitor YAT2150 has potent antimalarial activity in Plasmodium falciparum in vitro cultures: I.B. Arnaiz, et al.; BMC Biol.
20, 197 (2022),
Abstract;
ATP-citrate lyase promotes axonal transport across species: A. Even, et al.; Nat. Commun.
12, 5878 (2021),
Abstract;
Cell-Instructive Surface Gradients of Photoresponsive Amyloid-like Fibrils: A.M. Bender, et al.; ACS Biomater. Sci. Eng.
7, 4798 (2021),
Abstract;
Proteostasis regulated by testis-specific ribosomal protein RPL39L maintains mouse spermatogenesis: Q. Zou, et al.; iScience
24, 103396 (2021),
Abstract;
Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells: L. Liu, et al.; Nat. Biomed. Eng.
5, 1261 (2021),
Abstract;
Characterization of Protein Aggregates, Silicone Oil Droplets, and Protein-Silicone Interactions using Imaging Flow Cytometry: C. Probst; J. Pharm. Sci.
109, 364 (2020),
Abstract;
Endoplasmic reticulum chaperone calmegin is upregulated in aldosterone-producing adenoma and associates with aldosterone production: K. Itcho, et al.; Hypertension
75, 492 (2020),
Abstract;
Monitoring plasma protein aggregation during aging using conformation-specific antibodies and FTIR spectroscopy: S. Magalhaes, et al.; Clin. Chim. Acta
502, 25 (2020),
Application(s): Protein aggregation in human plasma samples,
Abstract;
PERK participates in cardiac valve development via fatty acid oxidation and endocardial-mesenchymal transformation: T. Shimizu, et al.; Sci. Rep.
10, 20094 (2020),
Abstract;
Full Text
The polyphenol quercetin protects from glucotoxicity depending on the aggresome in Caenorhabditis elegans: M. Civelek, et al.; Eur. J. Nutr.
59, 485 (2020),
Application(s): Protein aggregation in solution,
Abstract;
Fibril formation and therapeutic targeting of amyloid-like structures in a yeast model of adenine accumulation: D. Laor, et al.; Nat. Commun.
10, 62 (2019),
Abstract;
Full Text
Transthyretin amyloid fibril disrupting activities of extracts and fractions from Juglans mandshurica maxim. var. cordiformis (Makino) kitam: N. Chaudhary, et al.; Molecules
24, 500 (2019),
Application(s): Aged amyloid fibrils,
Abstract;
Full Text
Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway: Y.L. Wu, et al.; Free Radic. Biol. Med.
115, 309 (2018),
Abstract;
Discovering putative prion-like proteins in Plasmodium falciparum: A computational and experimental analysis: I. Pallares, et al.; Front. Microbiol.
9, 1737 (2018),
Application(s): P. falciparum culture,
Abstract;
Full Text
High Throughput Differential Scanning Fluorimetry (DSF) Formulation Screening with Complementary Dyes to Assess Protein Unfolding and Aggregation in Presence of Surfactants: S.M. McClure, et al.; Pharm. Res.
35, 81 (2018),
Abstract;
The catalytic inactivation of the N-half of human hexokinase 2 and structural and biochemical characterization of its mitochondrial conformation: M.H. Nawaz, et al.; BioSci. Rep.
38, BSR20171666 (2018),
Application(s): Aggregation propensity assessed by DSF,
Abstract;
Full Text
Aggregated transthyretin is specifically packaged into placental nano-vesicles in preeclampsia: M. Tong, et al.; Sci. Rep.
7, 6694 (2017),
Application(s): Protein aggregation in extracts from placental extracellular vesicles,
Abstract;
Full Text
Curcumin Improves Palmitate-Induced Insulin Resistance in Human Umbilical Vein Endothelial Cells by Maintaining Proteostasis in Endoplasmic Reticulum: M. Ye, et al.; Front. Pharmacol.
8, 148 (2017),
Abstract;
Full Text
Scaffold requirements for periodontal regeneration with enamel matrix derivative proteins: A. Apicella, et al.; Colloids Surf. B Biointerfaces
156, 221 (2017),
Application(s): Aggregation of enaml matrix derivative (EMD) proteins,
Abstract;
Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer's disease: E. Vion, et al.; Mol. Cell. Neurosci.
88, 1 (2017),
Abstract;
ARD1-mediated Hsp70 acetylation balances stress-induced protein refolding and degradation: J.H. Seo, et al.; Nat. Commun.
7, 12882 (2016),
Application(s): ,
Abstract;
Full Text
BRG1 and BRM SWI/SNF ATPases redundantly maintain cardiomyocyte homeostasis by regulating cardiomyocyte mitophagy and mitochondrial dynamics in vivo: S.J. Bultman, et al.; Cardiovasc. Pathol.
25, 258 (2016),
Abstract;
Cardiomyocyte-specific human Bcl2-associated anthanogene 3 P209L expression induces mitochondrial fragmentation, Bcl2-associated anthanogene 3: M.T. Quintana, et al.; Am. J. Pathol.
186, 1989 (2016),
Application(s): Lysates from cardiac tissues,
Abstract;
Full Text
Interfacial dilatational deformation accelerates particle formation in monoclonal antibody solutions: G.L. Lin, et al.; Soft Matter
12, 3293 (2016),
Abstract;
Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans: C. Regitz, et al.; Eur. J. Nutr.
55, 741 (2016),
Application(s): Protein aggregation in solution,
Abstract;
A screening methodology for purifying proteins with aggregation problems: M. Lebendiker, et al.; Methods Mol. Biol.
1258, 261 (2015),
Abstract;
Effects of tau domain-specific antibodies and intravenous immunoglobulin on tau aggregation and aggregate degradation: J.O. Esteves-Villanueva, et al.; Biochemistry
54, 293 (2015),
Abstract;
Metal-mediated protein oxidation: applications of a modified ELISA-based carbonyl detection assay for complex proteins: H. Uehara, et al.; Pharm. Res.
32, 691 (2015),
Application(s): Effects of metal-mediated protein oxidation on aggregation,
Abstract;
WALTZ-DB: a benchmark database of amyloidogenic hexapeptides: J. Beerten, et al.; Bioinformatics
31, 1698 (2015),
Abstract;
Amyloid-beta (Aβ1-42)-induced paralysis in Caenorhabditis elegans is reduced by restricted cholesterol supply: C. Regitz, et al.; Neurosci. Lett.
576, 93 (2014),
Abstract;
Human Stefin B Role in Cell's Response to Misfolded Proteins and Autophagy: M. Polajnar, et al.; PLoS One
9, e102500 (2014),
Application(s): Proteins aggregation and oxidative stress in stefin B KO astrocytes,
Abstract;
Full Text
Lysosomal enzyme cathepsin B enhances the aggregate forming activity of exogenous α-synuclein fibrils: A. Tsujimura, et al.; Neurobiol. Dis.
73C, 244 (2014),
Application(s): In vitro formation of a-synuclein fibrils and monitoring with ProteoStat® dye,
Abstract;
ProteoStat to detect and discriminate intracellular amyloid-like aggregates in Escherichia coli: S.Navarro & S.Ventura; Biotechnol. J
9, 1259 (2014),
Abstract;
Increased carbonylation, protein aggregation and apoptosis in the spinal cord of mice with experimental autoimmune encephalomyelitis: A. Dasgupta, et al.; ASN Neuro
5, e00111 (2013),
Application(s): Detection of protein aggregation in tissue section using fluorescence microscopy,
Abstract;
Full Text
Optimization of protein purification and characterization using Thermofluor screens: S. Boivin, et al.; Protein Expr. Pur.
91, 192 (2013),
Abstract;
Protein quality control acts on folding intermediates to shape the effects of mutations on organismal fitness: S. Bershtein, et al.; Mol. Cell.
49, 133 (2013),
Application(s): Propensity to aggregate for dihydrofolate reductase mutants,
Abstract;
Correction of both NBD1 energetics and domain interface is required to restore ΔF508 CFTR folding and function: W.M. Rabeh, et al.; Cell
148, 150 (2012),
Application(s): Aggregation of multi-domain protein: NBD1,
Abstract;
Full Text
Detection of α-synuclein amyloidogenic aggregates in vitro and in cells using light-switching dipyridophenazine ruthenium(II) complexes: N.P. Cook, et al.; J. Am. Chem. Soc.
134, 20776 (2012),
Abstract;
p62/SQSTM1-Dependent Autophagy of Lewy Body-Like α-Synuclein Inclusions: Y. Watanabe, et al.; PLoS One
7, e52868 (2012),
Application(s): Protein aggregation of alpha-synuclein,
Abstract;
Full Text
Raster image correlation spectroscopy as a novel tool for the quantitative assessment of protein diffusional behaviour in solution: Z. Hamrang, et al.; J. Pharm. Sci.
101, 2082 (2012),
Abstract;
High sensitivity luminescence nanoparticle assay for the detection of protein aggregation: S. Pihlasalo, et al.; Anal. Chem.
83, 1163 (2011),
Abstract;
General Literature References
Emerging analytical technologies for biotherapeutics development: R. Krishnamurthy et al.; Bioprocess International 6 (5), 32 (2008),
Aggregation analysis of therapeutic proteins, part 2: T. Arakawa et al.; Bioprocess International 5 (4), 36 (2007),
Aggregation analysis of therapeutic proteins, part 3: T. Arakawa et al.; Bioprocess International 5(10), 52 (2007),
Aggregation analysis of therapeutic proteins, part 1: T. Arakawa, et al.; Bioprocess International 4 (10), 32 (2006),
Structure of the cross-beta spine of amyloid-like fibrils: R. Nelson et al.; Nature
435, 773 (2005),
Abstract;
Related Products