-
Novel acidic organelle-selective dye, suitable for live cell staining of lysosomes
-
Long wavelength red emission that is easily multiplexed with common fluorescent dyes (coumarin, FITC, Cyanine 3) and fluorescent proteins (BFP, CFP, GFP, YFP)
-
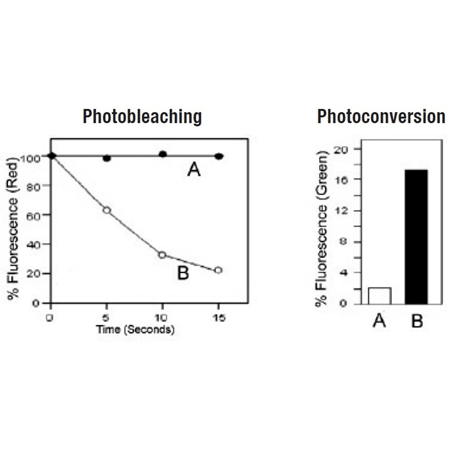
No photo conversion to a green emitting dye and no complicating metachromatic dual emission artifacts, facilitating analysis with GFP, FITC and other fluorescent probes
-
Highly resistant to photobleaching and concentration quenching, ensuring strong, consistent fluorescence signal, even after extended viewing periods
LYSO-ID® Red Lysosomal Detection Kit (GFP-CERTIFIED®) is an ideal choice for fluorescence co-localization imaging with GFP-tagged proteins, providing spectrally pure red signal. By contrast, many red-emitting lysosomal probes photoconvert to green fluorescent species or display metachromatic artifacts wherein emission occurs both in the red and green regions of the spectrum, leading to spurious results in GFP co-localization experiments [Freundt et al (2007) Cell Res. 17(11): 956- 958; Nadrigny et al (2007) Biophys J. 93(3):969-980]. The LYSO-ID® Red Lysosomal Detection Kit (GFP-CERTIFIED®)contains a proprietary acidic organelle-selective dye suitable for staining live cells. The dye is compatible with most fluorescence detection systems, including conventional and confocal fluorescence microscopes, as well as High Content Screening (HCS) platforms. This kit is specifically designed for use with GFP-expressing cell lines, as well as cells expressing blue, cyan or yellow fluorescent proteins (BFPs, CFPs, YFPs). The LYSO-ID® Red dye has been validated for utility in live cell imaging applications, showing an appropriate response to treatment with well characterized lysosomal perturbation agents. A lysosome perturbation agent, chloroquine, is provided as a positive control for monitoring changes in lysosome number and volume. A nuclear counterstain (Hoechst 33342) is provided to highlight this organelle as well.
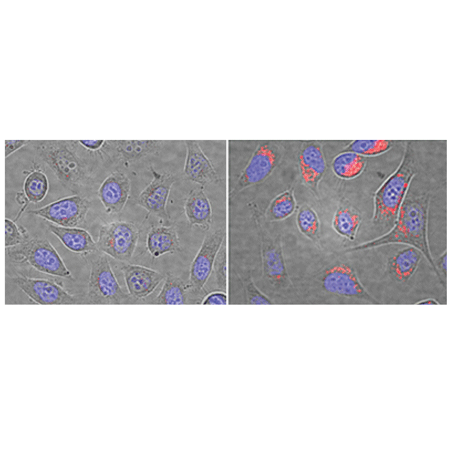
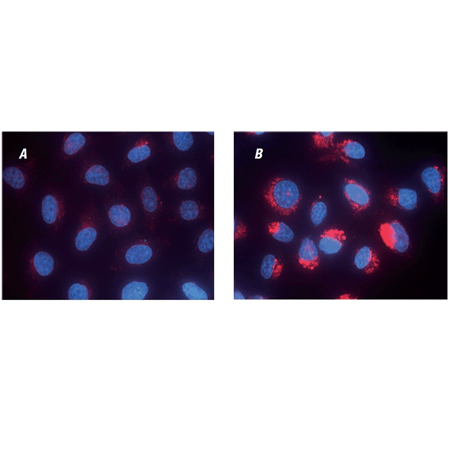
Composite bright-field and fluorescence microscopy images of control U2OS cells (left) and cells pre-treated with 64μM Chloroquine for 5 hours (right). Cells were stained with LYSO-ID® Red dye for 10 minutes. Nuclei were counter-stained with Hoechst 33342 dye.
Please mouse over
Product Details
| Applications: | Fluorescence microscopy, Fluorescent detection
|
| |
| Application Notes: | For use with GFP-expressing cell lines, as well as cells expressing blue, cyan or yellow fluorescent proteins. |
| |
| Quality Control: | A sample from each lot of LYSO-ID® Red detection kit (GFP-CERTIFIED®) is used to stain HeLa cells using the procedures described in the user manual. The chloroquine-treated HeLa cells exhibit dramatic increase in lysosome-like vesicle number and volume, comparing to untreated cells, when stained with LYSO-ID® Red Detection Reagent followed by microscopy analysis. |
| |
| Quantity: | -500 size contains reagents for 500 assays
-0100 size contains reagents for 100 assays |
| |
| Use/Stability: | With proper storage, the kit components are stable up to the date noted on the product label. Store kit at -20°C in a non-frost free freezer, or -80°C for longer term storage. |
| |
| Handling: | Avoid freeze/thaw cycles. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Contents: | LYSO-ID® Red Detection Reagent
Hoechst 33342 Nuclear Staining
Chloroquine Control
10X Assay Buffer |
| |
| Technical Info/Product Notes: | The LYSO-ID® red lysosomal detection kit (GFP-CERTIFIED® is a member of the CELLESTIAL® product line, reagents and assay kits comprising fluorescent molecular probes that have been extensively benchmarked for live cell analysis applications. CELLESTIAL® reagents and kits are optimal for use in demanding imaging applications, such as confocal microscopy, flow cytometry and HCS, where consistency and reproducibility are required.,
Cited samples: For an overview on cited samples please click here. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Product Literature References
A new missense variant in RAB3GAP2 in a family with muscular dystrophy-short stature and defective autophagy: An expansion of the micro/Martsolf spectrum or a new phenotype?: G.A. Mora-Roldan, et al.; Am. J. Med. Genet. A
188, 1972 (2022),
Abstract;
STING controls energy stress-induced autophagy and energy metabolism via STX17: Y. Rong, et al.; J. Cell Biol.
221, e202202060 (2022),
Abstract;
Modeling sialidosis with neural precursor cells derived from patient-derived induced pluripotent stem cells: B. Seol, et al.; Int. J. Mol. Sci.
22, 4386 (2021),
Abstract;
Full Text
Cathepsin inhibition modulates metabolism and polarization of tumor-associated macrophages: D. Oelschlaegel, et al.; Cancers (Basel)
12, 2579 (2020),
Abstract;
Full Text
Different sensitivity of macrophages to phospholipidosis induction by amphiphilic cationic drugs: K. Öhlinger, et al.; Int. J. Mol. Sci.
21, 8391 (2020),
Abstract;
Full Text
Lysosomal dysfunction and autophagy blockade contribute to MDMA-induced neurotoxicity in SH-SY5Y neuroblastoma cells: I.H. Li, et al.; Chem. Res. Toxicol.
33, 903 (2020),
Abstract;
1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) rapidly resolves LPS-induced acute lung injury through the effective control of neutrophil recruitment: H.R. Lee, et al.; Front. Immunol.
10, 2177 (2019),
Application(s): Fluorescence microscopy using Raw 264.7, HL-60, and HUVEC,
Abstract;
Anti-CotH3 antibodies protect mice from mucormycosis by prevention of invasion and augmenting opsonophagocytosis: T. Gebremariam, et al.; Sci. Rep.
5, eaaw1327 (2019),
Abstract;
Full Text
CXCR2 specific endocytosis of immunomodulatory peptide LL-37 in human monocytes and formation of LL-37 positive large vesicles in differentiated: Z. Zhang, et al.; Bone Rep.
12, 100237 (2019),
Application(s): Fluorescence microscopy,
Abstract;
Full Text
Drp1/Fis1 interaction mediates mitochondrial dysfunction in septic cardiomyopathy: B. Haileselassie, et al.; J. Mol. Cell. Cardiol.
130, 160 (2019),
Abstract;
Full Text
Immune homeostasis and regulation of the interferon pathway require myeloid-derived Regnase-3: M. von Gamm, et al.; J. Exp. Med.
216, 1700 (2019),
Abstract;
Full Text
Interferon inducible X-linked gene CXorf21 may contribute to sexual dimorphism in Systemic Lupus Erythematosus: C.A. Odhams, et al.; Sci. Rep.
10, 2164 (2019),
Abstract;
Full Text
Modulating immune response with nucleic acid nanoparticles: J.K. Durbin, et al.; Molecules
24, 3740 (2019),
Abstract;
Full Text
Ochratoxin A sequentially activates autophagy and the ubiquitin-proteasome system: H.A. Akpinar, et al.; Toxins (Basel)
11, 615 (2019),
Application(s): Confocal microscopy using HK-2 cells,
Abstract;
Full Text
Optimization and preclinical evaluation of novel histamine H3 receptor ligands: acetyl and propionyl phenoxyalkyl piperazine derivatives: K. Szczepanska, et al.; Bioorg. Med. Chem.
26, 6056 (2018),
Application(s): Fluorescence microscopy using HepG2 cells,
Abstract;
Spermine increases acetylation of tubulins and facilitates autophagic degradation of prion aggregates: K. Phadwal, et al.; Sci. Rep.
8, 10004 (2018),
Application(s): Confocal microscopy with CAD and SMB cells,
Abstract;
Full Text
Guanidinylated bioresponsive poly(amido amine)s designed for intranuclear gene delivery: J. Yu, et al.; Int. J. Nanomedicine
11, 4011 (2016),
Abstract;
Full Text
Induction of genomic instability and activation of autophagy in artificial human aneuploid cells: K. Ariyoshi, et al.; Mutat. Res.
790, 19 (2016),
Application(s): Fluorescence microscopy using human normal breast epithelial MCF-10A cells,
Abstract;
Novel guanidinylated bioresponsive poly(amidoamine)s designed for short hairpin RNA delivery: J. Yu, et al.; Int. J. Nanomedicine
11, 6651 (2016),
Application(s): Flow cytometry using MCF7 cells,
Abstract;
Full Text
VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington’s disease: X. Guo, et al.; Nat. Commun.
7, 12646 (2016),
Application(s): Immunocytochemistry to determine lysosomal activity,
Abstract;
WO3/Pt nanoparticles promote light-induced lipid peroxidation and lysosomal instability within tumor cells: A.J. Clark, et al.; Nanotechnology
27, 075103 (2016),
Abstract;
Analyzing the colocalization of MAP1LC3 and lysosomal markers in primary cells: K. Phadwal; Cold Spring Harb. Protoc.
2015, pdb.prot086272 (2015),
Abstract;
Autophagy limits proliferation and glycolytic metabolism in acute myeloid leukemia: A.S. Watson, et al.; Cell Death Discov.
1, Article number 15008 (2015),
Abstract;
Clathrin-dependent endocytosis of claudin-2 by DFYSP peptide causes lysosomal damage in lung adenocarcinoma A549 cells: A. Ikari, et al.; Biochim. Biophys. Acta
1848, 2326 (2015),
Abstract;
Identification and functional characterization of a solute carrier family 15, member 4 gene in Litopenaeus vannamei: Y. Chen, et al.; Dev. Comp. Immunol.
57, 57 (2015),
Application(s): Stained drosophila Schneider 2 (S2) cells,
Abstract;
Intracellular calcium levels as screening tool for nanoparticle toxicity: C. Meindl, et al.; J. Appl. Toxicol.
35, 1150 (2015),
Application(s): Fluorescent Detection,
Abstract;
Full Text
Retinoic acid-induced IgG production in TLR-activated human primary B cells involves ULK1-mediated autophagy: A.B. Eriksen, et al.; Autophagy
11, 460 (2015),
Application(s): Colocalization studies with anti-LC3B by confocal microscopy using human B cells,
Abstract;
SerpinB2 (PAI-2) Modulates Proteostasis via Binding Misfolded Proteins and Promotion of Cytoprotective Inclusion Formation: J.A. Lee, et al.; PLoS One
10, e0130136 (2015),
Application(s): Assay using murine embryonic fibroblasts,
Abstract;
Full Text
Threonine 56 phosphorylation of Bcl-2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy: Y.C. Su, et al.; Biochim. Biophys. Acta
1852, 12 (2015),
Abstract;
Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism: Y.H. Tseng, et al.; Autophagy
10, 20 (2014),
Abstract;
Macrophages eliminate circulating tumor cells after monoclonal antibody therapy: N. Gül, et al.; J. Clin. Invest.
124, 812 (2014),
Abstract;
Full Text
p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8⁺ T cells: S.M. Henson, et al.; J. Clin. Invest.
124, 4004 (2014),
Application(s): ImageStream analysis on PBMC,
Abstract;
Full Text
The c10orf10 gene product is a new link between oxidative stress and autophagy: M.W. Stepp, et al.; Biochim. Biophys. Acta
1843, 1076 (2014),
Application(s): Lysosomal detection in African green monkey kidney cells (VERO cells),
Abstract;
Epithelial uptake of flagella initiates proinflammatory signaling: D. Parker, et al.; PLoS One
8, e59932 (2013),
Application(s): Detection by confocal microscopy,
Abstract;
Full Text
Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation: Y.C. Su, et al.; Hum. Mol. Genet.
22, 4545 (2013),
Abstract;
Interactions between autophagic and endo-lysosomal markers in endothelial cells: C.L. Oeste, et al.; Histochem. Cell. Biol.
139, 659 (2013),
Abstract;
A novel method for autophagy detection in primary cells: Impaired levels of macroautophagy in immunosenescent T cells: K. Phadwal, et al.; Autophagy
8, 677 (2012),
Application(s): Lysosomal detection in PBMC using imaging flow cytometry,
Abstract;
Full Text
Cell death and autophagy under oxidative stress: roles of poly(ADP-Ribose) polymerases and Ca(2+): P. Wyrsch, et al.; Mol. Cell. Biol.
32, 3541 (2012),
Application(s): Lysosomal detection in mouse embryonic fibroblasts using fluorescence microscopy,
Abstract;
Full Text
Role of ATG8 and autophagy in programmed nuclear degradation in Tetrahymena thermophila: M.L. Liu, et al.; Eukaryot. Cell.
11, 494 (2012),
Application(s): Lysosomal detection in Tetrahymena using fluorescence microscopy,
Abstract;
Full Text
HspB8 Mutation Causing Hereditary Distal Motor Neuropathy Impairs Lysosomal Delivery of Autophagosomes: A.S. Kwok, et al.; J. Neurochem.
119, 1155 (2011),
Abstract;
A Live-Cell Fluorescence Microplate Assay Suitable for Monitoring Vacuolation Arising from Drug or Agent Treatment: J. Coleman, et al.; J. Biomol. Screen.
15, 398 (2010),
Abstract;
Related Products