Product Details
| Alternative Name: | Monophenol monooxygenase |
| |
| MW: | ~52kDa (SDS-PAGE) |
| |
| Source: | Produced in E. coli. |
| |
| EC: | 1.14.18.1 |
| |
| UniProt ID: | P14679 |
| |
| Formulation: | Liquid. In 50mM TRIS/HCl, pH 8.0, 1M NaCl, 0.05% Empigen BB, and 30% glycerol. |
| |
| Purity: | ≥85% (SDS-PAGE) |
| |
| Purity Detail: | Purified by multi-step chromatography. |
| |
| Activity: | One microgram of BML-SE535 will produce greater than 90pmol of adduct at 37°C over a period of 20 minutes using 0.5mM L-Tyrosine as substrate in 50mM potassium phosphate, pH 7.3, 2% dimethylformamide, 5mM MBTH (3-methyl-2-benzothiazolinone hydrazone) and monitoring formation of dopachrome-MBTH adduct at 484nm (ε484=22,300 M-1cm-1). NOTE: tyrosinases, in particular mammalian tyrosinase, are inherently inefficient enzymes, with typical changes in OD only in the deciOD range, although the reaction reaches maximal velocity quickly (typically within two minutes). In addition, its substrates and MBTH are very unstable; make stocks fresh and keep sealed in the dark until use. Lifetime and rate of enzymatic activity can be increased by the addition of CuCl to the assay buffer, but this will increase background and may complicate other redox reactions in the assay. |
| |
| Application Notes: | Useful tool to study enzyme kinetics and screen for inhibitors. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Handling: | Avoid freeze/thaw cycles. |
| |
| Scientific Background: | Tyrosinase is a copper-containing enzyme that catalyzes four steps in the melanin and catecholamine synthesis pathways. Like tyrosine hydroxylase (EC 1.13.16.2), it can catalyze the formation of L-DOPA in the brain and nervous system. It oxidizes monophenols, but like catecholoxidase (EC 1.10.3.1), it can also oxidize o-diphenols to o-quinones. It is involved in cancer, Parkinson’s, pigmentation diseases, Alzheimer’s and obesity. Much of the research and inhibitor screening has used mushroom tyrosinase. However, mushroom tyrosinase interacts very differently with substrates and inhibitors compared to the human enzyme. In addition, commercially available mushroom tyrosinase is not always of good purity. Recombinant versions of human tyrosinase amino acids 1-345 or 1-378, also available commercially, are not functional due the lack of critical catalytic and structural residues. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
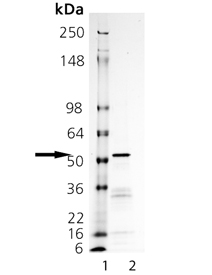
SDS-PAGE Analysis. Lane 1: MW Marker, Lane 2: 2 µg Tyrosinase.

Tyrosinase Activity: Formation of dopachrome-MBTH adduct monitoring at 484nm in a 96-well half area microwell plate with a range of enzyme loads. Maximum signal plateau was reached rapidly at 37°C, preventing an accurate time-dependent measurement.
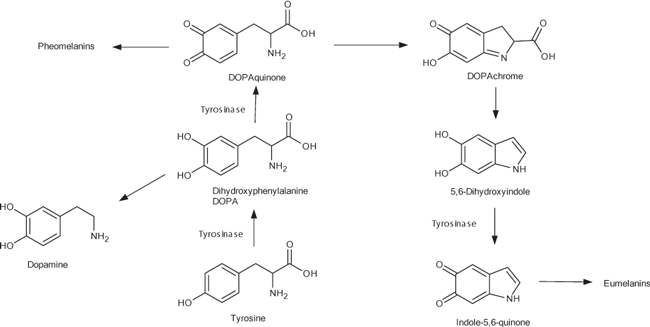
Melanin and catecholamine synthesis pathways
Please mouse over
Product Literature References
Optimization of amylomaltase for the synthesis of α-arbutin derivatives as tyrosinase inhibitors: P. Rudeekulthamrong & J. Kaulpiboon; Carbohydr. Res.
494, 108078 (2020),
Application(s): Test of inhibitors.,
Abstract;
3-hydroxycoumarin loaded vesicles for recombinant human tyrosinase inhibition in topical applications: M. Schlich, et al.; Colloids Surf. B Biointerfaces
171, 675 (2018),
Abstract;
Interaction of small molecules with human tyrosinase: A surface plasmon resonance and molecular docking study: S. Patil, et al.; Int. J. Biol. Macromol.
92, 1123 (2016),
Application(s): Enzyme activity assay; Surface plasmon resonance (SPR) studies; Screening and kinetic analysis; In silico docking,
Abstract;
Spontaneous cellular and humoral tumor antigen responses in patients with uveal melanoma: P.L. Triozzi, et al.; Melanoma Res.
25, 510 (2015),
Application(s): Added to PBMC cell culture,
Abstract;
The albino mutation of tyrosinase alters ocular angiogenic responsiveness: M. S. Rogers, et al.; Angiogenesis
16, 639 (2013),
Application(s): Added to cell culture to evaluate effect on endothelial cell migration.,
Abstract;
Full Text
General Literature References
Evidence for the ectopic synthesis of melanin in human adipose tissue: M. Randhawa, et al.; FASEB J.
23, 835 (2009),
Abstract;
Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes: S.R. Setty, et al.; Nature
454, 1142 (2008),
Abstract;
A. Rescigno, et al.; Enzyme Microb. Technol. 41, 620 (2007),
A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase: F. Garcia-Molina, et al.; J. Agric. Food Chem.
55, 9739 (2007),
Abstract;
A three-dimensional model of mammalian tyrosinase active site accounting for loss of function mutations: T. Schweikardt, et al.; Pigment Cell Res.
20, 394 (2007),
Abstract;
Discovery of small-molecule inhibitors of tyrosinase: J.P. Germanas, et al.; Bioorg. Med. Chem. Lett.
17, 6871 (2007),
Abstract;
Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries: S. Parvez, et al.; Phytother. Res.
21, 805 (2007),
Abstract;
Similar enzyme activation and catalysis in hemocyanins and tyrosinases: H. Decker, et al.; Gene
398, 183 (2007),
Abstract;
Regulation of tyrosinase trafficking and processing by presenilins: partial loss of function by familial Alzheimer’s disease mutation: R. Wang, et al.; PNAS
103, 353 (2006),
Abstract;
S.-H. Jeon, et al.; Bull. Korean Chem. Soc. 26, 1135 (2005),
Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease: M. Asanuma, et al.; Neurotox. Res.
5, 165 (2003),
Abstract;
Catecholamine synthesis is mediated by tyrosinase in the absence of tyrosine hydroxylase: M. Rios, et al.; J. Neurosci.
19, 3519 (1999),
Abstract;
Mutational mapping of the catalytic activities of human tyrosinase: R.K. Tripathi, et al.; J. Biol. Chem.
267, 23707 (1992),
Abstract;