Replaces Prod. #: ALX-300-146
Endogenous cannabinoid. Weak ligand of CB1 (Ki=23.8µM) and CB2 (Ki=13.9µM) receptor. Inhibits fatty acid amide hydrolase (FAAH) (IC50=5.1µM). Immunosuppressant, anti-inflammatory, anti-nociceptive and anti-convulsant in vivo. The exact mode of action has not yet been revealed. It has been suggested that PEA: i) binds to a yet to be discovered cannabinoid receptor similar to CB2; ii) administered in vivo elicits the synthesis of endogenous agonists of CB2; iii) acts as an "entourage" compound by enhancing the activity and/or by influencing the turnover of endogenous agonists of CB2, possibly but not uniquely, by inhibiting their degradation.
Product Details
| Alternative Name: | PEA, Palmidrol |
| |
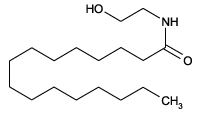
| Formula: | C18H37NO2 |
| |
| MW: | 299.5 |
| |
| CAS: | 544-31-0 |
| |
| Purity: | ≥98% (TLC) |
| |
| Appearance: | White solid. |
| |
| Solubility: | Soluble in DMSO or 100% ethanol. |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | +4°C |
| |
| Use/Stability: | Stable for at least 1 year after receipt when stored, as supplied, at 0-4°C. Stock solutions are stable for up to 3 months at -20°C. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals: G. Re, et al.; Vet. J.
173, 21 (2007), Review,
Abstract;
Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems: L. De Petrocellis, et al.; Clin. Pharmacol.
16, 297 (2002),
Abstract;
The palmitoylethanolamide family: a new class of anti-inflammatory agents?: D.M. Lambert, et al.; Curr. Med. Chem.
9, 663 (2002),
Abstract;
Effects of homologues and analogues of palmitoylethanolamide upon the inactivation of the endocannabinoid anandamide: K.O. Jonsson, et al.; Br. J. Pharmacol.
133, 1263 (2001),
Abstract;
Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB(2) cannabinoid, as potential ligands for the cannabinoid receptors: D.M. Lambert, et al.; Biochim. Biophys. Acta
1440, 266 (1999),
Abstract;
Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP: H. Cadas, et al.; J. Neurosci.
16, 3934 (1996),
Abstract;
N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation: S. Mazzari, et al.; Eur. J. Pharmacol.
300, 227 (1996),
Abstract;
The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons: S.D. Skaper, et al.; PNAS
93, 3984 (1996),
Abstract;
Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide: L. Facci, et al.; PNAS
92, 3376 (1995),
Abstract;
Isolation and structure of a brain constituent that binds to the cannabinoid receptor: W.A. Devane, et al.; Science
258, 1946 (1992),
Abstract;