Replaces Prod. #: ALX-380-108
Antibiotic. Potent and specific inhibitor of mitochondrial complex II (succinate-ubiquinone oxidoreductase).
Product Details
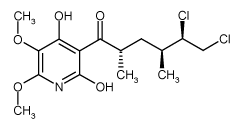
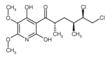
| Alternative Name: | 3-[(2S,4S,5R)-5,6-Dichloro-2,4-dimethyl-1-oxohexyl]-4-hydroxy-5,6-dimethoxy-2(1H)-pyridinone |
| |
| Formula: | C15H21Cl2NO5 |
| |
| MW: | 366.2 |
| |
| Source: | Synthetic. Originally isolated from Penicillium sp. strain FO-125. |
| |
| CAS: | 119509-24-9 |
| |
| RTECS: | CJ8800000 |
| |
| Purity: | ≥95% (HPLC) |
| |
| Appearance: | White to off-white powder. |
| |
| Solubility: | Soluble in acetone, acetonitrile, chloroform, ethyl acetate, DMSO, methanol or 100% ethanol; insoluble in water or hexane. |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | -20°C |
| |
| Technical Info/Product Notes: | The IC50 value against bovine heart complex II is 3.6nM (which is ~300-fold lower than the IC50 value of carboxin (1.1μM)). It also inhibits fumarate reductase of Ascaris suum (IC50 = 12nM). Inhibition of E. coli succinate dehydrogenase is less potent (IC50 = 5μM).By co-crystallization studies of atpenin A5 and complex II, the binding site of atpenin A5 was found to be the quinone-binding site of complex II. Additionally, atpenin A5 has been shown to have a protective action against ischemia-reperfusion via the activation of mitochondrial KATP channels. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
Ym155 localizes to the mitochondria leading to mitochondria dysfunction and activation of AMPK that inhibits BMP signaling in lung cancer cells: A. Mondal, et al.; Sci. Rep.
12, 13135 (2022),
Abstract;
BCPP compounds, PET probes for early therapeutic evaluations, specifically bind to mitochondrial complex I: S. Kazami, et al.; Mitochondrion
46, 97 (2019),
Abstract;
Dual loss of succinate dehydrogenase (SDH) and complex I activity is necessary to recapitulate the metabolic phenotype of SDH mutant tumors: D. Lorendeau, et al.; Metab. Eng.
43(Pt B), 187 (2017),
Abstract;
Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation: K. Kluckova, et al.; Cell Death Dis.
6, e1749 (2015),
Application(s): Cell Culture,
Abstract;
Full Text
Enantioselective total synthesis of atpenin A5: M. Ohtawa et al.; J. Antibiot.
62, 289 (2009),
Abstract;
The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels: A. P. Wojtovich & P. S. Brooks; Basic Res. Cardiol.
104, 121 (2009),
Abstract;
Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction: R. Horsefield, et al.; J. Biol. Chem.
281, 7309 (2006),
Abstract;
Full Text
Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase): H. Miyadera, et al.; PNAS
100, 473 (2003),
Abstract;
Mechanism of action of atpenin B on Raji cells: K. Oshino, et al.; J. Antibiot.
43, 1064 (1990),
Abstract;
The structures of atpenins A4, A5 and B, new antifungal antibiotics produced by Penicillium sp: H. Kumagai, et al.; J. Antibiot.
43, 1553 (1990),
Abstract;
Atpenins, new antifungal antibiotics produced by Penicillium sp. Production, isolation, physico-chemical and biological properties: S. Omura, et al.; J. Antibiot.
41, 1769 (1988),
Abstract;