Replaces Prod. #: BML-EI207
Selenium-based inhibitor of protein kinase C, NADPH, 5-lipoxygenase, cyclooxygenase (COX) and NADPH oxidase. Anti-inflammatory antioxidant. Mimics glutathione peroxidase. Inhibits oxidative modifications of low density lipoproteins (LDL). Protects cerebellar granule neurons against 4-hydroxynonenal-induced neuronal death.
Product Details
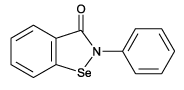
| Formula: | C13H9NOSe |
| |
| MW: | 274.2 |
| |
| CAS: | 60940-34-3 |
| |
| Purity: | ≥98% (HPLC) |
| |
| Identity: | Determined by NMR. |
| |
| Appearance: | White to off-white crystalline solid. |
| |
| Solubility: | Soluble in DMSO or 100% ethanol. |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | -20°C |
| |
| Handling: | Protect from light. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
A general covalent binding model between cytotoxic selenocompounds and albumin revealed by mass spectrometry and X-ray absorption spectroscopy: W. Zheng, et al.; Sci. Rep.
10, 1274 (2020),
Abstract;
Full Text
Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation: S. Hamarsheh, et al.; Nat. Commun.
11 , 1659 (2020),
Abstract;
Full Text
Quantitative high-throughput screening identifies cytoprotective molecules that enhance SUMO conjugation via the inhibition of SUMO-specific protease (SENP)2: J.D. Bernstock, et al.; FASEB J.
32, 1677 (2018),
Abstract;
Differential induction of ATF3 and HO-1 in myeloid cells and keratinocytes via Dimethylfumarate or Cyclosporine A: S. Müller, et al.; J. Dermatol. Sci.
87, 246 (2017),
Application(s): Treatment of human keratinocytes and PBMCs,
Abstract;
Reactive oxygen species formation during tetanic contractions in single isolated Xenopus myofibers: L. Zuo, et al.; J. Appl. Physiol.
111, 898 (2011),
Abstract;
Full Text
Ebselen protects mice against T cell-dependent, TNF-mediated apoptotic liver injury: G. Tiegs, et al.; J. Pharmacol. Exp. Ther.
287, 1098 (1998),
Abstract;
Full Text
Protection against peroxynitrite by selenoproteins: H. Sies, et al.; Z. Naturforsch. [C]
53, 228 (1998),
Abstract;
Ebselen as a glutathione peroxidase mimic and as a scavenger of peroxynitrite: H. Sies & H. Masumoto; Adv. Pharmacol.
38, 229 (1997),
Abstract;
Free radical generation by selenium compounds and their prooxidant toxicity: J.E. Spallholz; Biomed. Environ. Sci.
10, 260 (1997),
Abstract;
Ebselen: H. Sies; Meth. Enzymol.
252, 341 (1995),
Abstract;
Molecular actions of ebselen-an antiinflammatory antioxidant: T. Schewe; Gen. Pharmacol.
26, 1153 (1995),
Abstract;
Ebselen: a glutathione peroxidase mimic: H. Sies; Meth. Enzymol.
234, 476 (1994),
Abstract;
Effects of ebselen and probucol on oxidative modifications of lipid and protein of low density lipoprotein induced by free radicals: N. Noguchi, et al.; Biochim. Biophys. Acta
1213, 176 (1994),
Abstract;
Interaction of ebselen with glutathione S-transferase and papain in vitro: T. Nikawa, et al.; Biochem. Pharmacol.
47, 1007 (1994),
Abstract;
Strong inhibition of mammalian lipoxygenases by the antiinflammatory seleno-organic compound ebselen in the absence of glutathione: C. Schewe, et al.; Biochem. Pharmacol.
48, 65 (1994),
Abstract;
Action of ebselen as an antioxidant against lipid peroxidation:: N. Noguchi, et al.; Biochem. Pharmacol.
44, 39 (1992),
Abstract;
Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C?: I.A. Cotgreave, et al.; Biochem. Pharmacol.
38, 649 (1989),
Abstract;
Kinetic mechanism and substrate specificity of glutathione peroxidase activity of ebselen (PZ51): M. Maiorino, et al.; Biochem. Pharmacol.
37, 2267 (1988),
Abstract;
Seleno-organic compounds and the therapy of hydroperoxide-linked pathological conditions: M.J. Parnham & E. Graf; Biochem. Pharmacol.
36, 3095 (1987),
Abstract;
A novel biologically active seleno-organic compound--III. Effects of PZ 51 (Ebselen) on glutathione peroxidase and secretory activities of mouse macrophages: M.J. Parnham & S. Kindt; Biochem. Pharmacol.
33, 3247 (1984),
Abstract;
General Literature References
Ebselen is a new skin depigmenting agent that inhibits melanin biosynthesis and melanosomal transfer: B. Kasraee, et al.; Exp. Dermatol.
21, 19 (2012),
Abstract;