Replaces Prod. #: BML-PI105
Irreversible inhibitor of cysteine proteinases like papain, calpain, cathepsin B, L and H. Acts by forming a thioether bond with the thiol of the active cysteine. Does not inhibit serine proteinases. Inhibits degradation of autophagic cargo in autophagolysosomes.
Product Details
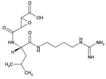
| Alternative Name: | N-[N-(L-3-trans-Carboxyoxirane-2-carbonyl]-L-leucyl)-agmatine, L-trans-Epoxysuccinyl-leucylamide-(4-guanido)-butane |
| |
| Formula: | C15H27N5O5 . 0.5 H20 |
| |
| MW: | 357.4 |
| |
| Source: | Synthetic. |
| |
| CAS: | 66701-25-5 |
| |
| Purity: | ≥98% |
| |
| Appearance: | White to off-white solid. |
| |
| Solubility: | Soluble in water/ethanol 1:1 mixture (15 mg/ml). |
| |
| Shipping: | Ambient Temperature |
| |
| Long Term Storage: | -20°C |
| |
| Handling: | Protect from light. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
Please mouse over
Product Literature References
Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape: J. Dinter, et al.; PLoS Pathog.
11, e1004725 (2015),
Application(s): Cell Culture,
Abstract;
Full Text
Efficient synthetic method for ethyl (+)-(2S,3S)-3-[(S)-3-methyl- 1-(3-methylbutylcarbamoyl)butylcarbamoyl]-2-oxiranecarb oxylate (EST), a new inhibitor of cysteine proteinases: M. Tamai, et al.; Chem. Pharm. Bull. (Tokyo)
35, 1098 (1987),
Abstract;
Isolation and Characterization of E-64, a New Thiol Protease Inhibitor: K. Hanada, et al.; Agric. Biol. Chem.
42, 523 (1978),
Full Text
Structure and Synthesis of E-64, a New Thiol Protease Inhibitor: K. Hanada, et al.; Agric. Biol. Chem.
42, 529 (1978),
Full Text