Product Details
| Alternative Name: | Chaperonin 60, CPN60, HspD1, Heat shock protein 60 |
| |
| Recommended Dilutions/Conditions: | Western Blot (100ng, colorimetric)
Suggested dilutions/conditions may not be available for all applications.
Optimal conditions must be determined individually for each application. |
| |
| MW: | ~60kDa |
| |
| Source: | Produced in E. coli. |
| |
| UniProt ID: | P10809 |
| |
| Formulation: | Liquid. In Dulbecco's PBS. |
| |
| Purity: | ≥90% (SDS-PAGE; Western blot) |
| |
| Purity Detail: | Purified by multi-step chromatography. |
| |
| Applications: | WB
Activity assay
|
| |
| Application Notes: | ATPase activity assay (positive). Western blot control. |
| |
| Shipping: | Dry Ice |
| |
| Long Term Storage: | -80°C |
| |
| Scientific Background: | The human Hsp60 is a member of a highly conserved family which includes molecular chaperones from several species including plant Hsp60 (known as Rubisco binding protein), and bacterial GroEL, a major antigen of mycobacteria. In eukaryotes, Hsp60 is localized in the mitochondrial matrix while plant Hsp60 is localized in the chloroplast. Mitochondria, chloroplasts and bacteria have a common ancestry (>1billion years). This fact combined with the high degree of homology between the divergent Hsp60s would indicate that these proteins carry out a primitive but important function which is conserved in divergent species. The common characteristics of the Hsp60s include i) high abundance, ii) induction upon environmental stress such as heat shock, iii) homo-oligomeric structures of either 7 or 14 subunits which reversibly dissociate in the presence of Mg (2+) and ATP, iv) ATPase activity and v) a role in folding and assembly of oligomeric protein structures. These similarities are supported by studies where the single-ring human mitochondrial homolog, Hsp60 with its co-chaperonin, Hsp10 were expressed in anE. coli strain, engineered so that the groE operon is under strict regulatory control. This study has demonstrated that expressed Hsp60-Hsp10 was able to carry out all essential in vivo functions of GroEL and its co-chaperonin, GroES. Consistent with their function as chaperones, Hsp60 and Hsp10 have been suggested to act as docking molecules with a passive role in the maturation of caspase processing. Recombinant Hsp60 and Hsp10 have been shown to accelerate the activation of procaspase-3 by cytochrome c and d ATP in an ATP-dependent manner. Hsps are intracellular proteins which are thought to serve protective functions against infection and cellular stress, however several studies indicate that members of the Hsp60 family are linked to a number of autoimmune diseases, artherosclerosis and chlamydial disease. |
| |
| Regulatory Status: | RUO - Research Use Only |
| |
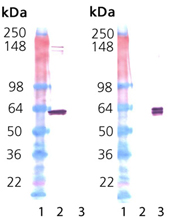
Western Blot Analysis: Lane 1: MWM; Lane 2: 100 ng Hsp60 protein (Prod. No. ADI-SPP-540); Lane 3: 100 ng GroEL protein (Prod. No. ADI-SPP-610); Left: probed with Hsp60 mAb (Prod. No. ADI-SPA-806) at 1.0 μg/ml. Right: probed with GroEL mAb (Prod. No. ADI-SPS-870) at 1.0 μg/ml.
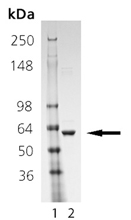
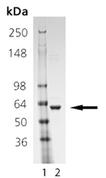
SDS-PAGE Analysis: Lane 1: MWM; Lane 2: 1.0 µg of purified Human Hsp60 protein.
Please mouse over
Product Literature References
Hsp60 expression profiles in the reef-building coral Seriatopora caliendrum subjected to heat and cold shock regimes: D. Seveso, et al.; Mar. Environ. Res.
119, 1 (2016),
Application(s): Western blot,
Abstract;
Up-regulation of Hsp60 in response to skeleton eroding band disease but not by algal overgrowth in the scleractinian coral Acropora muricata: D. Seveso, et al.; Mar. Environ. Res.
78, 34 (2012),
Abstract;
General Literature References
A single-ring mitochondrial chaperonin (Hsp60-Hsp10) can substitute for GroEL-GroES in vivo: K.L. Nielsen, et al.; J. Bacteriol.
181, 5871 (1999),
Abstract;
Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells: A. Samali, et al. ; EMBO J.
18, 2040 (1999),
Abstract;
Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen: S. Jindal, et al.; Mol. Cell Biol.
9, 2279 (1989),
Abstract;
Related Products